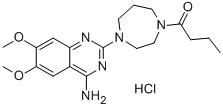
Manufacturing Process
17.0 g of 2-chloro-4-amino-6,7-dimethoxyquinazoline and 18.2 g of Nformylhomopiperazine
are added to 170 ml of butanol and the whole is
refluxed with stirring for 3 h. After completion of the reaction, the mixture is
cooled, and the crystals thus precipitated are filtered out, washed with a small
quantity of ethanol and air-dried. 25.0 g of crude 2-homopiperazino-4-amino-
6,7-dimethoxyquinazoline are obtained.
A solution of 2-homopiperazino-4-amino-6,7-dimethoxyquinazoline in 60 ml of
acetone is added dropwise to a solution of 2-butylcarboxylic acid chloride in
acetone under stirring and ice-cooling. After completion of the addition, the
stirring is continued for additional 1 h to complete the reaction. The crystals
thus precipitated are filtered out and the 1-(4-amino-6,7-dimethoxy-2-
quinazolinyl)hexahydro-4-(1-oxobutyl)-1H-1,4-diazepine, melting point 280-
282°C (recrystallized from a mixture of methanol-ethanol) is obtained.