Description
Thiodicarb is a white crystalline powder with a slight sulphurous odour. Thiodicarb is stable in light and ambient conditions and unstable in alkaline conditions. Thiodicarb is a carbamate insecticide. Thiodicarb is commonly used to protect agricultural crops from major lepidopterous insect pests and suppresses coleopterous and some hemipterous insect pests. Thiodicarb acts as an ovicide against cotton bollworms and budworms. Thiodicarb is used primarily on cotton, sweet corn, and soybeans. Thiodicarb is formulated to include several liquid products and one powdered product that must be mixed with water before field application. Thiodicarb is reclassified as an RUP. Thiodicarb degrades rapidly to methomyl, which is already a restricted use chemical.
References
http://www.wisegeek.com/what-is-thiodicarb.htm
http://pmep.cce.cornell.edu/profiles/insect-mite/propetamphos-zetacyperm/thiodicarb/insect-prof-thiodicarb.html
http://www.inchem.org/documents/jmpr/jmpmono/v00pr09.htm#_00092120
Description
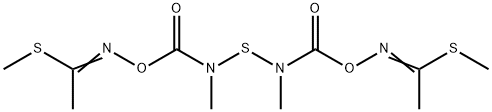
Thiodicarb, 3,7,9,13-tetramethyl-5,11-
dioxa-2,8,14-trithia-4,7,9,12-tetra-azapentadeca-3,12-di
ene-6,10-dione (IUPAC), consists of colorless crystals, which
are sparingly soluble in water, readily soluble in
dichloromethane, acetone, methanol, and xylene.
Thiodicarb is produced by reaction of N,N -thiobis(methylcarbamic
acid fluoride) with 2-methylthioacetaldoxim in the presence of a base.
Uses
Thiodicarb is an oxime carbamate insecticide and ovicide with
both oral and contact activities against major Lepidoptera, Coleoptera,
Diptera and Hemiptera pests in/on cotton, maize, fruits, soyabeans and
vegetables.
Uses
Thiodicarb is used as an insecticide.
General Description
Colorless to pale tan crystals. Non corrosive. Used as an insecticide.
Air & Water Reactions
Hydrolyzed by strong acid or base.
Reactivity Profile
A carbamate derivative. Carbamates are chemically similar to, but more reactive than amides. Like amides they form polymers such as polyurethane resins. Carbamates are incompatible with strong acids and bases, and especially incompatible with strong reducing agents such as hydrides. Flammable gaseous hydrogen is produced by the combination of active metals or nitrides with carbamates. Strongly oxidizing acids, peroxides, and hydroperoxides are incompatible with carbamates.
Agricultural Uses
Insecticide, Molluscicide, Ovicide: Not approved for use in EU countries. Registered
for use in the U.S. Thiodicarb is used primarily on cotton,
sweet corn, and soybeans. The remaining usage is spread
among leafy vegetables, cole crops, ornamentals, and
other minor use sites. Thiodicarb acts as an ovicide against
cotton bollworms and budworms.
Trade name
CGA® 45156; CHIPCO[C];
DICARBOSULF®; DICARBASULF®; LARVIN®;
LEPICRON®; SEMEVIN®; NIVRAL®; UC-51762®;
UC 51769®; UC 80502®
Environmental Fate
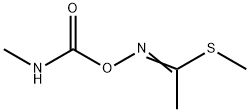
Soil. Under aerobic and anaerobic soil conditions, thiodicarb degrades to methomyl
and methomyl oxime (Hartley and Kidd, 1987). The reported half-life in various soils is
3–8 days (Hartley and Kidd, 1987).
Metabolic pathway
The initial metabolic reaction of thiodicarb in soils, plants and animals
is the hydrolytic or thiolytic cleavage of the N-S bond to methomyl.
The subsequent metabolic pathway of methomyl involves hydrolysis /
elimination reactions to yield S-methyl-N-hydroxythioacetimidate and
ultimately acetonitrile and CO
2 as the major terminal products. The metabolic
pathways of thiodicarb are presented in Scheme l. See also the
methomyl entry.
Degradation
Thiodicarb (1) is susceptible to alkaline hydrolysis (Feung and Heinzelmann,
1989). Thiodicarb was stable between pH 5 and 6, but it degraded
rapidly in alkaline conditions (pH 9) with a DT
50 of less than one day. The
DT
50 values of thiodicarb at pH 3 and 7 were 9 and 13 days, respectively.
The initial degradation product of thiodicarb was methomyl (2) which
was further hydrolysed to S-methyl-N-hydroxytoacetimidate (3) in
alkaline solution (pH 9).
Photolysis of thiodicarb in water was not significant (Andrawes
and College, 1977). The photolytic DT
50 of thiodicarb was approximately
81 days. The major photolytic degradation product was methomyl(2).