Synthesis
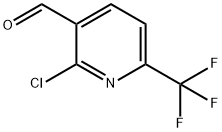
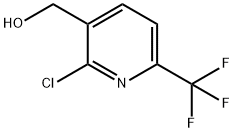
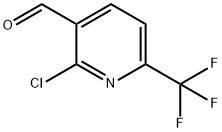
General procedure for the synthesis of 2-chloro-6-(trifluoromethyl)nicotinaldehyde from (2-chloro-6-(trifluoromethyl)pyridin-3-yl)methanol: In a 100 mL round bottom flask, add (2-chloro-6-(trifluoromethyl)pyridin-3-yl)methanol (1.00 g, 4.73 mmol), dichloromethane (10 mL), PCC (2.04 g, 9.45 mmol. Aldrich) and silica gel (6.00 g). The reaction mixture was stirred at room temperature for 3 hours before being filtered through a pad of silica gel and washed with a 4:1 solvent mixture of ethyl acetate/hexane. The filtrate was concentrated under vacuum to give 2-chloro-6-(trifluoromethyl)nicotinaldehyde (0.805 g, 81% yield) as a colorless oil.1H NMR (CDCl3) δ 10.50 (s, 1H), 8.42 (d, J = 7.8 Hz, 1H), 7.80 (d, J = 7.8 Hz, 1H).
References
[1] Patent: WO2010/83246, 2010, A1. Location in patent: Page/Page column 105-106
[2] Patent: WO2013/64460, 2013, A1. Location in patent: Page/Page column 127