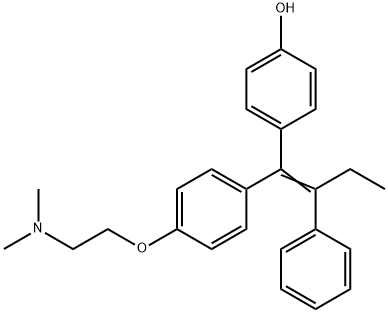
Chemical Properties
Off-White Solid
Uses
A selective estrogen receptor modulator.
Uses
(E/Z)-4-Hydroxy Tamoxifen is selective estrogen receptor modulator.
Definition
ChEBI: Afimoxifene is a tertiary amino compound that is tamoxifen in which the phenyl group which is in a Z- relationship to the ethyl substituent is hydroxylated at the para- position. It is the active metabolite of tamoxifen. It has a role as an antineoplastic agent, an estrogen receptor antagonist and a metabolite. It is a tertiary amino compound and a member of phenols. It is functionally related to a tamoxifen.
brand name
TamoGel (Ascend Therapeutics).
General Description
4-Hydroxytamoxifen is a first generation, selective estrogen receptor modulator (SERM) that functions as an antagonist in breast cancer cells but can display estrogen-like activities in the uterus and bone.
Biological Activity
4-hydroxytamoxifen is an estrogen receptor modulator.estrogen receptor can be selectively stimulated or inhibited, providing promising therapeutic opportunities for auto-immune diseases, prostate and breast cancer, as well as depression.
Biochem/physiol Actions
Metabolite of the chemotherapeutic drug tamoxifen, exhibiting more potent estrogen agonist/antagonist activity than the parent drug. Also active as intra-membranous inhibitor of lipid peroxidation.
in vitro
previous study was conducted to evaluate the effects of tamoxifen and its active metabolite 4-hydroxytamoxifen on isolated rat cardiac myocyte mechanical function and calcium handling. results showed that myocytes treated with 4-hydroxytamoxifen had similarly to tamoxifen-treated cells to both calcium handling and contractility [1].
in vivo
previous animal study compared the extent of dna adduct formation in sd rats treated with seven tamoxifen or 4-hydroxytamoxifen. results showed that the liver weights and microsomal rates were not changed by tamoxifen or 4-hydroxytamoxifen treatment. moreover, the uterine weights were significantly decreased and uterine peroxidase activity was marginally decreased in tamoxifen or 4-hydroxytamoxifen treated rats. in addition, hepatic dna adduct levels in rats treated with 4-hydroxytamoxifen did not differ from control rats. similaryly, the adduct levels in uterus dna from rats treated with tamoxifen or 4-hydroxytamoxifen were not different from those in control rats [2].
IC 50
27 and 18 μm for mcf-7 and mda-mb-231 cell proliferation
References
[1] asp ml,martindale jj,metzger jm. direct, differential effects of tamoxifen, 4-hydroxytamoxifen, and raloxifene on cardiac myocyte contractility and calcium handling. plos one.2013 oct 24;8(10):e78768.
[2] beland fa,mcdaniel lp,marques mm. comparison of the dna adducts formed by tamoxifen and 4-hydroxytamoxifen in vivo. carcinogenesis.1999 mar;20(3):471-7.
[3] lee o et al. a randomized phase ii presurgical trial of transdermal 4-hydroxytamoxifen gel versus oral tamoxifen in women with ductal carcinoma in situ of the breast. clin cancer res.2014 jul 15;20(14):3672-82.