Manufacturing Process
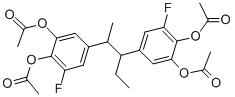
4.5 g erythro-3,3'-difluoro-4,4'-dihydroxy-α-ethyl-α'-methylbibenzyl in 30 ml
pyridine and 10 ml acetic anhydride were left to stand for 24 h at room
temperature, thereafter the reaction mixture was worked up to give a crude
product, recrystallisation of which from chloroform-diethyl ether (1:4 v/v)
gave 5.5 g erythro-3,3'-difluoro-4,4'-diacetoxy-α-ethyl-α'-methylbibenzyl,
melting point 118°-120°C.
5.0 g of the erythro-3,3'-difluoro-4,4'-diacetoxy-α-ethyl-α'-methylbibenzyl
were ground with 10.0 g aluminum chloride and the mixture was heated at
150°C for 30 min. After cooling, the mass was added portion-wise to ice,
stirred and extracted with chloroform. The chloroform solution was washed
with water, dried with anhydrous sodium sulfate and concentrated to half its
volume. An equal volume of diethyl ether was added thereto and the solution
was treated with charcoal and filtered. The filtrate was evaporated and the
residue obtained was crystallized from chloroform-diethyl ether (1:1 v/v) to
give 2.6 g erythro-3,3'-diacetyl-4,4'-dihydroxy-5,5'-difluoro-α-ethyl-α'-
methylbibenzyl, melting point 194°-196°C.
0.9 g erythro-3,3'-diacetyl-4,4'-dihydroxy-5,5'-difluoro-α-ethyl-α'-
methylbibenzyl were suspended in a mixture of 10 ml dioxan and 6 ml 1 N
aqueous sodium hydroxide solution. The solution was cooled to 10°C and 0.8
ml 30% hydrogen peroxide added dropwise thereto. The reaction mixture was
stirred at 20°C for 1.5 h and then poured into a mixture of dilute hydrochloric
acid and ice. The product was extracted with peroxide-free diethyl ether, the
extract was evaporated and the residue was crystallized from benzene to give
a yellow solid. This was treated in 5 ml ethanol with 2.0 mg sodium
borohydride, followed by acidification with hydrochloric acid, rapid extraction
with diethyl ether, evaporation of the extract and immediate crystallization
from benzene to give 350.0 mg of pure erythro-4,4',5,5'-tetrahydroxy-3,3'-
difluoro-α-ethyl-α'-methyldibenzyl, melting point 143°-145°C.
1.0 g erythro-3,3'-difluoro-4,4',5,5'-tetrahydroxy-α-ethyl-α'-methyldibenzyl in
a mixture of 10 ml pyridine and 5 ml acetic anhydride was left to stand at
20°C for 20 h and at 60°-80°C for 1 h. The reaction mixture was then poured
into a mixture of dilute hydrochloric acid and ice, stirred for several h and
filtered. The filtrate was evaporated and the residue was taken up in chloroform and the chloroform solution was washed with dilute hydrochloric
acid and water, dried over anhydrous sodium sulfate and the solvent removed.
The gummy residue was taken up with benzene and the benzene solution
filtered through a neutral alumina. The benzene was removed from the filtrate
to give 1.0 g pure erythro-3,3'-difluoro-4,4',5,5'-tetraacetoxy-α-ethyl-α'-
methyldibenzyl; melting point 160°-162°C (crystallised from diethyl ether).