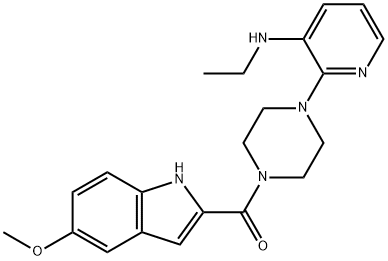
atevirdine
- Product Nameatevirdine
- CAS136816-75-6
- CBNumberCB91074739
- MFC21H25N5O2
- MW379.46
- MDL NumberMFCD00866956
- MOL File136816-75-6.mol
atevirdine Price
| Product number | Packaging | Price | Product description | Buy |
|---|---|---|---|---|
| American Custom Chemicals Corporation API0010741 | 5MG | $504.6 | ATEVIRDINE 95.00% |
Buy |
atevirdine Chemical Properties,Usage,Production
Originator
Atevirdine,UpjohnUses
Antiviral.Manufacturing Process
1-[5-Methoxyindolyl-2-carbonyl]-4-[3-(ethylamino)-2-pyridinyl]piperazine:1,1'-Carbonyldiimidazole (0.55 g) is added to a 20°-25°C solution of 5- methoxyindole-2-carboxylic acid (0.59 g) in tetrahydrofuran (7.0 ml). After stirring 1 hour, the reaction is transferred via cannnula into a solution of 1-(3- N-ethylamino-2-pyridinyl)piperazine (0.70 g) in tetrahydrofuran (7 ml) at - 12°C (ice/acetone bath). The reaction is stirred at -10°C for 30 minutes, then slowly warmed to 20°-25°C and stirred a further 18 hours. After diluting with ether (60 ml), the mixture is washed with saturated aqueous sodium bicarbonate (70 ml), saline (70 ml) and dried over anhydrous sodium sulfate. The mixture is concentrated under reduced pressure to a residue which is purified by flash chromatography (2 cm x 20 cm) eluting with methanol/chloroform (2/98) to give the title compound, mp 153°-154°C.
1-[5-Methoxyindolyl-2-carbonyl]-4-[3-(ethylamino)-2-pyridinyl]piperazine hydrochloride:
1-(Ethyl)-3-(dimethylaminopropyl)carbodiimide (1.25 g) is added to a solution of 1-(3-ethyl-2-pyridinyl)piperazine (1.12 g) in THF (15 ml). The reaction is stirred at 20°-25°C for 3 hr, then it is dissolved in chloroform (50 ml) and extracted with saturated aqueous sodium bicarbonate, saline, dried over anhydrous sodium sulfate and concentrated under reduced pressure. Purification by flash column chromatography (200 g silica) eluting with ethyl acetate/hexane (50/50), the appropriate fractions are pooled and concentrated to give the title compound, The product is dissolved in methanol (150 ml) with heating, cooled to 20°-25°C and chlorotrimethylsilane (4.70 mmol) is added. The mixture is concentrated to half-volume, ether is added until cloudy and the flask is stored at 0°C overnight. Filtration gives the hydrochloride salt, MP: 194°-195°C.
The mesylate salt is formed by dissolving the free base in methanol and by adding methanesulfonic acid (1 eq). The solution is diluted with diethyl ether until the salt crystallizes out of solution. The crystals are collected and dried to afford the mesyl salt of the title compound, MP: 215°-216°C.
Therapeutic Function
AntiviralToxics Screening Level
The initial threshold screening level (ITSL) for atevirdine mesylate is 16 μg/m3 based on an annual averaging time.Preparation Products And Raw materials
atevirdine Suppliers
Global(9)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| +undefined-21-51877795 | ivan@atkchemical.com | China | 32965 | 60 | |
| 1-(800)-881-8210 | inquiries@lgmpharma.com | United States | 2123 | 70 | |
| +86057186818502 13588463833 |
info@sagechem.com | China | 10266 | 58 | |
| 021-QQ:65489617 15618227136 |
Sales@ATKchemical.com | China | 7312 | 58 | |
| 63313187 15358055255 |
63313187@qq.com | China | 1988 | 58 | |
| 4008200310 | marketing@tsbiochem.com | China | 24644 | 58 | |
| 021-65675885 18964387627 |
customer_service@efebio.com | China | 11974 | 58 | |
| 15052267297 15052267297 |
630819472@qq.com | China | 69 | 58 |
136816-75-6, atevirdineRelated Search
PROMPT×
PROMPT
The What'sApp is temporarily not supported in mainland China
The What'sApp is temporarily not supported in mainland China
Cancel
Determine