Synthesis
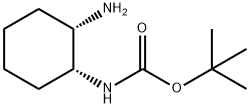
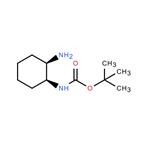
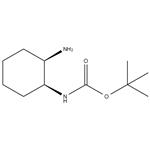
Triphenylphosphine (14.3 g) was added to a THF (190 ml) solution containing tert-butyl S,2S)-2-hydroxycyclohexyl) carbamate (10.0 g), followed by ice cooling. Diethyl azodicarboxylate (40% in toluene) (24.3 g) and DPPA (15.3 g) were added dropwise to the reaction solution, followed by stirring at room temperature for 1 hour. The reaction solution was left overnight. The solvent was distilled away under reduced pressure. Water was added, and then a 20% sodium hydroxide aqueous solution was added. Then, the organic layers were collected. Water (30 ml) was added to the obtained organic layers, followed by heating to 60 C. A THF (40 ml) solution containing triphenylphosphine (14.3 g) was added dropwise, followed by reflux for 2.5 hours. The solvent was distilled away under ordinary pressure. Toluene was added, and the pH was adjusted with 3M hydrochloric acid to pH=1 or less. Then, the resulting aqueous layers were collected, ethyl acetate was added, and the pH was adjusted with a 20% sodium hydroxide aqueous solution to pH 12. The organic layers were collected and dried over anhydrous sodium sulfate. Light yellow oily matter of tert-butyl ((1S,2R)-2-aminocyclohexyl)carbamate (Carbamic acid, [(1S,2R)-2-aminocyclohexyl]-, 1,1-dimethylethyl ester (9CI))was thus obtained.
![Carbamic acid, [(1S,2R)-2-aminocyclohexyl]-, 1,1-dimethylethyl ester (9CI) Structure](https://www.chemicalbook.com/CAS/GIF/365996-30-1.gif)