Manufacturing Process
5.6 ml of triethylamine are added to a solution of 5.0 g (0.018 m) of α-
methyl-α,α-dibromo-o-xylene and 2.38 g (0.018 m) of t-butylcarbazate in 15
ml of dimethylformamide heated to 50-60°C. After the addition, the mixture is
left stirring for 3 h at room temperature, the solution volume is then made up
to about 60 ml by diluting with H 2 O and the solution is left for a further hour
under stirring. The solid which separates is filtered off, washed with water and
dried to give 3.14 g (70%) of 1-methyl-N-(t-butyloxycarbonylamino)
isoindoline, melting point 143-145°C.
A suspension of 2.6 g (0.0104 m) of the 1-methyl-N-(t-
butyloxycarbonylamino)isoindoline in 7 ml of concentrated HCl is kept stirring
at room temperature for 1 h. The final solution is evaporated to dryness under
vacuum by heating to 60-70°C to give a solid residue (1.97 g) which
crystalises from EtOH+Et 2 O (1/1) to give 1.5 g (77.6%) of 1-methyl-2-amine
isoindoline hydrochloride, melting point 140-145°C.
3.44 g (0.0 135 m) of 4-chloro-3-sulfamidobenzoic acid chloride are added to
a solution of 2.5 g (0.0135 m) of 1-methyl-2-aminoisoindoline hydrochloride
and 3.5 g (0.0314 m) of triethylamine in 30 ml of tetrahydrofuran. The
mixture is kept stirring at room temperature for 15 h. The abundant solid
which separates is filtered off, and is suspended in water in order to remove
the triethylamine hydrochloride present. The residue is collected by filtration
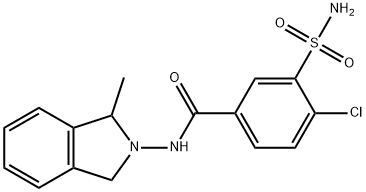
and dried in a drier to give 3.0 g of 1-methyl-2-(3'-sulfamyl-4'-
chlorobenzamido)isoindoline, melting point 208-210°C (the analytical sample
crystallizes from 10 volumes of ethyl alcohol, and has a melting point of 210-
212°C).
A further amount (0.4 g) of product can be isolated by evaporating the
tetrahydrofuran reaction solution, mixing the oily residue with ethyl alcohol
and allowing it to crystallize in a refrigerator. The overall yield is 3.4 g, equal
to 68.6%.