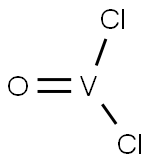
Chemical Properties
Green, deliquescent crystal. Usual technical
product is a dark-green, syrupymass, 76–82%
pure, or a solution. Slowly decomposed by water;
soluble in water, alcohol, and acetic acid; may react
violently with potassium.
Uses
Vanadium Dichloride Oxide has been used as mordant in printing fabrics.
Preparation
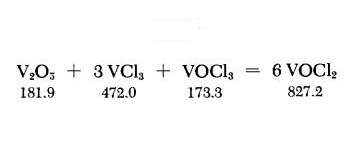
A thoroughly ground mixture of 3.6 g. of dry V2O5 and 9.4 g. of VCl3 is placed at the closed end of a l-m.-longtube, and 0.9 ml. of VOCl3 is then added. The upper part of the tube must be free of traces of these substances. The tube, filled with air, is melt-sealed, and is covered along its entire length with a sheet-metal jacket; its lower third, in a slightly inclined position, is then heated to about 600℃ with a tubular electric furnace. The sheet-metal jacket provides a temperature gradient along which the product VOCl2 sublimes out of the hot reaction zone. This procedure requires at least 4-5 days. However, the yield can be increased by longer heating time. Green needlelike crystals of VOCl2 are deposited in the cold part of the tube. The tube is opened at a suitable spot; the product is suspended in petroleum ether, ethyl ether or CCl4 to dissolve some adhering VOCl3, and then suction-filtered on a coarse fritted-glass filter. The relatively coarse filter separates the VOCl2 crystals from traces of finely divided hydrolysis products. The VOCl2 is freed of adhering solvent and stored under anhydrous conditions.
Hazard
Strong irritant to tissue.