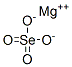Description
Magnesium selenate has the molecular formula of
MgSeO4 and the molecular weight of 167.2566 g/mol.
It can be prepared by reacting sodium selenate with
magnesium chloride in solution:
MgCl2(aq)+ Na2SeO4(aq) ? MgSeO4+ 2NaCl(aq)
It is soluble in water and the solution must be evaporated
to obtain crystals of the hexahydrate,
MgSeO4·6H2O. Its molecular weight is 275.3641 g/mol.
It is a white, monoclinic crystal with a density of
1.928 g/cm3. Its CAS number is 13446-58-1 and it is
soluble in water (55.52 g/100 ml at 60°C). The anhydrate
is stable upon heating to 1338°C the heat of formation,
△H
f°= —968.5 kJ/mol.