Originator
Pulsan,Yamanouchi,Japan,1979
Manufacturing Process
(a) A mixture of 0.9 g of 4-hydroxyindene, 2.0 g of 1,2-epoxy-3-
chloropropane (epichlorohydrin), 2.7 g of potassium carbonate and 15 ml of
acetone was refluxed at about 57°C for 24 hours. Acetone was removed by
vacuum distillation, the residue was washed with 10 ml of water and then
extracted with 20 ml of ether three times. The ether extract was dried with
magnesium sulfate, filtered and subjected to column chromatography using a
column (having an inside diameter of about 3 cm and a height of about 50
cm) packed with silica gel. The 5th to 7th fractions (volume of one fraction is
50 ml) recovered from the chromatographic column using chloroform as the
effluent were combined together and concentrated to provide 0.6 g of 4-(2,3-
epoxypropoxy)indene.
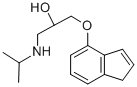
(6) A mixture of 0.42 g of 4-(2,3-epoxypropoxy)indene, 1.20 g of
isopropylamine and 20 ml of methanol was stirred in a flask at room
temperature for 2 hours. Methanol and unchanged isopropylamine were
removed by vacuum distillation and the residue was recrystallized from a
mixture of n-hexane and ether to yield 0.41 g of 4-(3-isopropylamino-2-
hydroxypropoxy)indene having a melting point of 88°C to 89°C.
(c) To a solution of 0.41 g of 4-(3-isopropylamino-2-hydroxypropoxy)indene in
80 ml of absolute ether there was added dropwise a hydrochloric acid-ether
mixture at 0°C with stirring. The precipitates thus formed were recovered by
filtration and recrystallized from a mixture of ethanol and ether to provide
0.44 g of the hydrochloride of 4-(3-isopropylamino-2-hydroxypropoxy)indene.
Melting point 147°C to 148°C.