Chemical Properties
colourless liquid
Chemical Properties
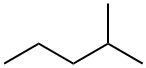
3-Methylpentane, C6H14, is a colorless, flammable liquid
with specific gravity 0.664. It occurs in petroleum and
natural gas, and may be released to the environment in
evaporative losses, wastewater, spills, and combustion
exhaust.
Physical properties
Clear, colorless, flammable liquid with an odor similar to hexane, heptane, and similar aliphatic
hydrocarbons. An odor threshold concentration of 8.9 ppm
v was reported by Nagata and Takeuchi
(1990).
Uses
3-Methylpentane is used as a solvent in the preparation of vegetable oils, glues, coatings, and paints. It is also used in gasoline, rubber solvent, and petroleum ether.
Uses
3-Methylpentane is a component of three typical commercial
hexanes, obtained from the fractionation of
natural gas liquids, a refinery operation involving hydrogenation,
and a stream meeting polymerization.
Other than for fuel, it is used in extraction of oil from
seeds and as a solvent and reaction medium in the manufacture
of polyolefins, synthetic rubbers, and some
pharmaceuticals.
Uses
Organic synthesis, solvent.
Definition
ChEBI: 3-methylpentane is an alkane that is pentane which is substituted by a methyl group at position 3. It is used as a solvent in organic synthesis, as a lubricant and as a raw material for producing carbon black. It has a role as a human metabolite, an allelochemical and a non-polar solvent. It is an alkane and a volatile organic compound.
Hazard
Flammable, dangerous fire risk.
Safety Profile
May have narcotic or
anesthetic properties. A very dangerous fire
hazard when exposed to heat or flame; can
react vigorously with oxidizing materials.
Explosive in the form of vapor when
exposed to heat or flame. To fight fire, use
foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and
irritating fumes.
Source
Schauer et al. (1999) reported 3-methylpentane in a diesel-powered medium-duty truck
exhaust at an emission rate of 670 μg/km.
California Phase II reformulated gasoline contained 3-methylpentane at a concentration of 22.7
g/kg. Gas-phase tailpipe emission rates from gasoline-powered automobiles with and without
catalytic converters were 3.76 and 512 mg/km, respectively (Schauer et al., 2002).
Environmental Fate
Photolytic. The following rate constants were reported for the reaction of 3-methylpentane and
OH radicals in the atmosphere: 4.30 x 10
-9 cm
3/molecule?sec at 300 K (Darnall et al., 1976); 6.8 x
10
-12 cm
3/molecule?sec at 305 K (Darnall et al., 1978); 5.7 x 10
-12 cm
3/molecule?sec (Altshuller,
1991).
Chemical/Physical. Complete combustion in air produces carbon dioxide and water vapor. 3-
Methylpentane will not hydrolyze because it does not contain a hydrolyzable functional group.
Purification Methods
Purify it by azeotropic distillation with MeOH, as for 2-methylpentane. Purify it for ultraviolet spectroscopy by passing it through columns of silica gel or alumina activated by heating for 8hours at 210o under a stream of nitrogen. Alternatively treat it with conc (or fuming) H2SO4, then wash it with water, aqueous 5% NaOH, water again, then dry (CaCl2, then sodium), and distil it through a long, glass helices-packed, column. [Beilstein 1 IV 363.]
Toxics Screening Level
The initial threshold screening level (ITSL) for 3-methylpentane is 3,500 μg/m3 based on an 8 hr. averaging time.