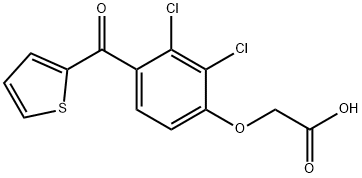
Description
Cytochrome P450 enzymes function to metabolize both endogenous and exogenous compounds. Both the 3A and 2C isoforms are present in human liver of which CYP2C9 seems most highly expressed. Tienilic acid is a specific suicide substrate for CYP2C9 and CYP2C10 whereby its oxidation inactivates the enzyme. The time required to inactivate one half of the CYP2C10 enzyme at the maximal rate (t
½max) is 3.4 minutes, with a dissociation constant (KI) value of 4.3 μM.
Chemical Properties
Pale Beige Solid
Uses
A heterocycloc derivative of phenoxyacetic Acid. Uses as a diuretic, uricosuric, anhtihypertensive
Uses
Tienilic Acid is a heterocyclic derivative of phenoxyacetic acid. Tienilic acid is used as diuretic, uricosuric, antihypertensive.
Definition
ChEBI: An aromatic ketone that is 2,3-dichlorophenoxyacetic acid in which the hydrogen at position 4 on the benzene ring is replaced by a thiophenecarbonyl group. A loop diuretic used to treat hypertension, it was withdrawn from the market in 1982 due to links wi
h hepatitis.
brand name
Tienilic Acid is INN and BAN;Anp 3624;Diflarex;Fr 3068;Selcryn;Skf-62698;Ticrynafen;Ticrynapen.
World Health Organization (WHO)
Tienilic acid, a diuretic agent with uricosuric and antihypertensive
activity, was introduced in 1976. By 1979 its use had been associated with cases of
hepatic toxicity, some of which were fatal, which led to the withdrawal of the drug
in most countries in which it was marketed. In France, however, precautions
regarding the use of tienilic acid were issued by the Pharmacovigilance
Commission and the drug remained available for another decade. In 1991, it was
eventually also withdrawn there since cases of hepatitis, some of which were
fulminant, had continued to occur.
Biochem/physiol Actions
Tienilic Acid (Ticrynafen) is a P450 inhibitor, a specific suicide substrate for CYP2C9 and CYP2C10. It was once used as a loop diuretic drug with uric acid-lowering (uricosuric) actvity, but was removed from market because of hepatotoxicity.