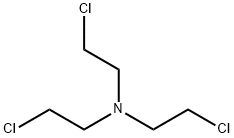
Description
Nitrogen mustards are very potential chemical substances of yesteryears and produced during the 1920s and 1930s as chemical warfare weapons. They are vesicants (or blister agents) similar to the sulphur mustards. They smell fishy, musty, soapy, or fruity and are either in the form of an oily textured liquid or a vapour (the gaseous form of a liquid) or a solid. It is in liquid form at normal room temperature (70 F) with a clear, pale amber, or yellow colour. HN-1, HN-2, and HN-3 are the military designations of nitrogen mustard (for more data, refer to Muatars gas). Nitrogen mustards (HN-1, HN-2, HN-3) are colourless to yellow, oily liquids that evaporate very slowly. HN-1 has a faint fishy or musty odour. HN-2 has a soapy odour at low concentrations and a fruity odour at higher concentrations. HN-3 may smell like butter almond. Use of nitrogen mustards is very much restricted other than for chemical warfare. In fact, presently, its use has no records. HN-1 has been used to remove warts in the past, and HN-2 has been used sparingly in chemotherapy.
Chemical Properties
HN-3, a nitrogen mustard blister agent (vesicants,
is a colorless to pale yellow liquid. Pure material is
odorless; otherwise, it has a faint fish-or soap-like odor.
General Description
Liquid with faint odor of fish and soap, no odor when pure. Used as a delayed-action casualty military agent.
Air & Water Reactions
Acts as a strong base in water.
Reactivity Profile
When dissolved in water, TRIS-(2-CHLOROETHYL)-AMINE is a strong base; reacts violently with strong oxidants and acids ; attacks copper and copper compounds. [Handling Chemicals Safely 1980. p. 123]; reacts with hypochlorites to give N-chloroamines, some of which are explosives when isolated [Bretherick 1979. p. 108].
Health Hazard
Most toxic of the nitrogen mustards. The median lethal dose for inhalation is 1,500 mg-min/m3; for skin absorption (masked personnel) is 10,000 mg-min/m3. The medium incapacitating dose for eye injury is 200 mg-min/m3; for skin absorption is 2,500 mg-min/m3.
Fire Hazard
When heated to decomposition, TRIS-(2-CHLOROETHYL)-AMINE emits chloride and nitrogen oxides. No action on metals or other materials if material is kept dry. Avoid decomposing heat.
Potential Exposure
Trichlorotriethylamine is a (slowacting)
vesicant but has never been used in military conflict.
Is used as an antineoplastic agent. Has been tested as
a fixing agent in textile dyes.
Carcinogenicity
Tris(β-chloroethyl)amine was
tested for carcinogenic potential in mice and rats given
the material by subcutaneous injection. The study in mice
was considered inadequate for evaluation by the IARC.
However, in rats, this material induced a high incidence of
sarcomas (mostly spindle-cell type) in animals of each sex
at the site of injection, as well as a few intestinal adenocarcinomas;
neither tumor type was seen in controls.
Intravenous, intraperitoneal, and subcutaneous injection
to mice and rats produced tumors in 15–20% of treated
animals. The tumors included fibrosarcomas, lymphosarcomas,
and adenocarcinomas, and administration of a
single dose was as effective in inducing tumors as multiple
injections.
IARC considered the preceding data “sufficient evidence
of carcinogenicity in experimental animals.” Although no
human carcinogenicity data were available, the IARC classified
tris(β-chloroethyl)amine in Group 2B (the agent is
possibly carcinogenic to humans). This classification was
based on these animal studies, its mutagenic potential, and
analogy to other nitrogen mustards.
Shipping
UN2810 Toxic liquids, organic, n.o.s., Hazard
Class: 6.1; Labels: 6.1-Poisonous materials, Technical
Name Required. Military driver of HN-3 shall be given full
and complete information regarding shipment and conditions
in case of emergency. AR 50-6 deals specifically with
the shipment of chemical agents. Shipments of agent will
be escorted in accordance with AR 740-32.
Incompatibilities
HN-3 is not stable; it undergoes slow but
steady polymerization. Avoid contamination with oxidizers
(chlorates, nitrates, peroxides, permanganates, perchlorates,
chlorine, bromine, fluorine, bleaches and pool chemicalsetc);
contact may cause fires or explosions. Keep away from alkaline
materials, strong bases, strong acids, oxoacids, epoxides.
Unstable in the presence of light and heat and forms dimers
at temperatures above 50℃. HN-3 decomposes before its
boiling point is reached or condenses under all conditions;
the reactions involved could generate enough heat to cause
an explosion. Polymerizes slowly, so munitions would be
effective for several years. Heated to decomposition emits
hydrogen chloride and nitrogen oxide. Note: Chlorinating
agents destroy nitrogen mustards. Dry chlorinated lime and
chloramines with a high content of active chlorine vigorously
chlorinate nitrogen mustards to the carbon chain giving
low toxicity products. In the presence of water this interaction
proceeds less actively. They are rapidly oxidized by peracids
in aqueous solution at weakly alkaline pH. In acid
solution the oxidation is much slower.