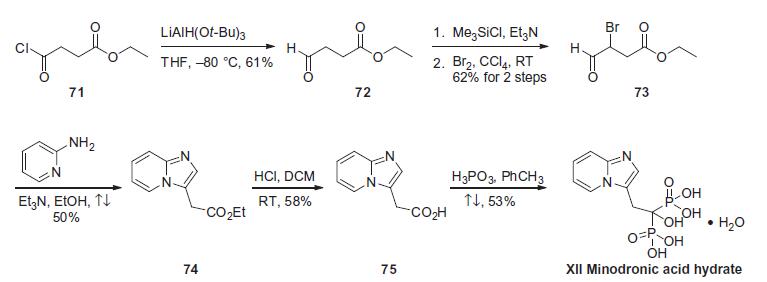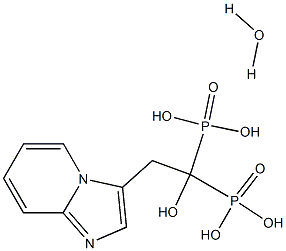
Minodronic Acid
- Product NameMinodronic Acid
- CAS155648-60-5
- CBNumberCB82521286
- MFC9H14N2O8P2
- MW340.163582
- EINECS696-656-6
- MDL NumberMFCD00890772
- MOL File155648-60-5.mol
Chemical Properties
| storage temp. | 2-8°C |
| Water Solubility | Insoluble in water |
| solubility | 0.1 M NaOH: 2mg/mL, clear (warmed) |
| form | powder to crystal |
| color | White to Light yellow to Light red |
| InChI | InChI=1S/C9H12N2O7P2.H2O/c12-9(19(13,14)15,20(16,17)18)5-7-6-10-8-3-1-2-4-11(7)8;/h1-4,6,12H,5H2,(H2,13,14,15)(H2,16,17,18);1H2 |
| InChIKey | GPAPAOGRNKUFGH-UHFFFAOYSA-N |
| SMILES | C(P(O)(O)=O)(O)(P(O)(O)=O)CC1N2C(=NC=1)C=CC=C2.[H]O[H] |
| FDA UNII | 457X74V7ND |
| UNSPSC Code | 12352106 |
| NACRES | NA.77 |
Safety
| Symbol(GHS) |
 
|
| Signal word | Danger |
| Hazard statements | H301-H361 |
| Precautionary statements | P201-P202-P264-P270-P280-P301+P310+P330-P308+P313-P405-P501 |
| RIDADR | 2811 |
| RTECS | SZ8562470 |
| HS Code | 2933.99.8290 |
| HazardClass | 6.1 |
| PackingGroup | III |