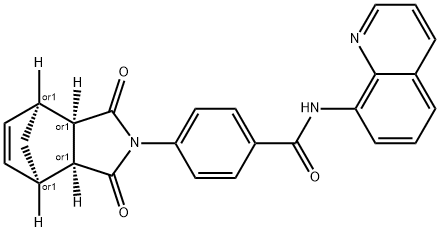
Description
IWR-1 endo (1127442-82-3) is a potent inhibitor of Wnt signaling (IC50=180 nM).1?Inhibits zebrafish tailfin regeneration (0.5 mM).2?Acts via inhibition of tankyrase and attenuates Wnt/β-catenin signaling in cancer stem-like cells.3?Promotes self-renewal and maintains pluripotency of human embryonic stem cells.4?Promotes differentiation of pluripotent stem cells into cardiomyocytes.5
Uses
IWR-3 act as inhibitors of Wnt response. It appear that IWR compounds induce stabilization of Axin proteins via a direct interaction, which is a part of the b-catenin destruction complex (consists of Apc, Axin, Ck1 and Gsk3b).Such compounds may be used in the treatment of Wnt protein signaling-related diseases and conditions such as cancer, degenerative diseases, type II diabetes and osteopetrosis.
Uses
IWR-1-endo act as inhibitors of Wnt response. It appear that IWR compounds induce stabilization of Axin proteins via a direct interaction, which is a part of the b-catenin destruction complex (consists of Apc, Axin, Ck1 and Gsk3b). Such compounds may be used in the treatment of Wnt protein signaling-related diseases and conditions such as cancer, degenerative diseases, type II diabetes and osteopetrosi s.
Definition
ChEBI: IWR-1-endo is a dicarboximide having an endo bridged phthalimide structure, substituted at nitrogen by a 4-(quinolin-8-ylcarbamoyl)benzoyl group. It has a role as an axin stabilizer and a Wnt signalling inhibitor. It is a dicarboximide, a bridged compound, a member of quinolines and a member of benzamides.
General Description
A cell-permeable
p-imidobenzamidoquinoline,
endo-diastereomer that is shown to inhibit the activity of TNKS1/PARP5a and TNKS2/PARP5b in
in vitro auto-PARsylation assays (IC
50 = 131 and 56 nM, respectively) and effectively suppress Wnt-stimulated transcription activity in L-Wnt-STF-based reporter assays (IC
50 = 180 nM), while exhibiting little activity against PARP1 or PARP2 (IC
50 >18.75 μM). Although both IWR-1-
endo and XAV939 (Tankyrase1/2 Inhibitor;
>Cat. No. 575545) act as reversible Wnt pathway inhibitors and exhibit similar pharmacological effects both
in vitro and
in vivo, IWR-1-
endo exerts its effect via interaction with Axin, while XAV939 binds TNKS directly.
Biochem/physiol Actions
Cell permeable: yes
References
1) Chen?et al. (2009),?Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer; Nature Chem. Biol.,?5?100
2) Lu?et al.?(2009),?Structure-activity Relationship Studies of Small-Molecule Inhibitors of Wnt Response; Bioorg. Med. Chem. Lett.,?19?3825
3) Martins-Neves?et al.?(2018),?IWR-1, a tankyrase inhibitor, attenuates Wnt/?-catenin signaling in cancer stem-like cells and inhibits in vivo the growth of subcutaneous human osteosarcoma xenograft; Cancer Lett.,?414?1
4) Kim?et al.?(2013),?Modulations of ?-catenin function maintains mouse epiblast stem cell and human embryonic stem cell self-renewal; Nat. Commun., 4?4403
5) Ren?et al.?(2011),?Small Molecule Wnt Inhibitors Enhance the Efficiency of BMP-4-directed Cardiac Differentiation of Human Pluripotent Stem Cells; J. Mol. Cell. Cardiol.,?51?280