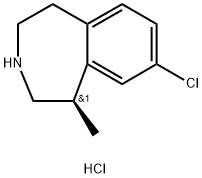
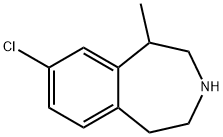
Description
In June 2012, the US FDA approved lorcaserin for chronic weight management
in adult patients who are characterized as overweight or obese and
have at least one comorbid, weight-related condition.
Lorcaserin (also known as APD356) is the first 5-HT
2C agonist
approved for chronic weight management since the withdrawal of the
5-HT
2C agonist fenfluramine in 1997 due to rare cases of cardiac valvulopathy. The serotonin receptor 5-HT
2C is found primarily in the hypothalamus
and regulates appetite and feeding behavior. Safety issues with
fenfluramine were associated with poor selectivity for 5-HT
2C versus
5HT
2A and 5HT
2B. Ring constraint afforded by the benzazepine in
lorcaserin improved selectivity for 5HT
2C by over 2 log units.
Description
Lorcaserin (hydrochloride) (CRM) (Item No. 19247) is a certified reference material categorized as an anorectic. It also decreases oxycodone intake and has positive effects on self-administration and relapse vulnerability in the rat. Lorcaserin is regulated as a Schedule IV compound in the United States. This product is intended for research and forensic applications.
Originator
Pharmaceuticals (United States)
Uses
Lorcaserin hydrochloride, a novel antiobesity drug, is a selective serotonin 5-HT2C receptor agonist, approved by FDA in 2012.
Definition
ChEBI: A hydrochloride obtained by reaction of lorcaserin with one equivalent of hydrochloric acid. Used as an anti-obesity drug.
Side effects
Lorcaserin hydrochloride (Belviq) is a serotonin 2C receptor agonist that causes weight loss by causing appetite suppression.It appears to have fewer adverse effects than orlistat,although long-term safety data are limited. The recommended dosage is 10mg twice daily. Lorcaserin should be discontinued if patients do not lose 5% of their body weight in 12 weeks. The efficacy of lorcaserin appears similar to that of orlistat (mean difference in weight loss between active and placebo-treated groups approximately 3-4 kg).Common side effects include nausea, headache,dizziness, nasopharyngitis, and fatigue. Lorcaserin may increasethe risk of symptomatic hypoglycemia in patients with type 2 diabetes on oral agents, necessitating a reduction in the dose of diabetes medications. Lorcaserin should not be used in individuals with creatinine clearance <30 mL/minute. It is contraindicated during pregnancy. In addition, lorcaserin should not be used with other serotonergic drugs (e.g., selective serotonin reuptake inhibitors,selective serotonin-norepinephrine reuptake inhibitors, bupropion[Wellbutrin], tricyclic antidepressants,and monamine oxidase inhibitors)because of the theoretical potential for serotonin syndrome.
https://www.accessdata.fda.govhttps://medlineplus.gov
Drug interactions
Belviq (lorcaserin hydrochloride) may interact with antidepressants, including selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), monoamine oxidase inhibitors (MAOIs), triptans, bupropion, dextromethorphan, or St. John's Wort.
Precautions
The precautions of BELVIQ (lorcaserin hydrochloride) tablets:
Serotonin Syndrome or Neuroleptic Malignant Syndrome (NMS)- like Reactions: The safety of coadministration with other serotonergic or antidopaminergic agents has not been established.
Valvular heart disease: If signs or symptoms develop, consider BELVIQ discontinuation and evaluate the patient for possible valvulopathy.
Cognitive Impairment: This may cause disturbances in attention or memory. Caution with the use of hazardous machinery when starting BELVIQ treatment.
Psychiatric Disorders, including euphoria and dissociation: Do not exceed the recommended dose of 10 mg twice daily.
Monitor for depression or suicidal thoughts. Discontinue if symptoms develop.
Use of Antidiabetic Medications: Weight loss may cause hypoglycemia. Monitor blood glucose. BELVIQ has not been studied in patients taking insulin.
Priapism: Patients should seek emergency treatment if an erection lasts >4 hours. Use BELVIQ with caution in patients predisposed to priapism.