Manufacturing Process
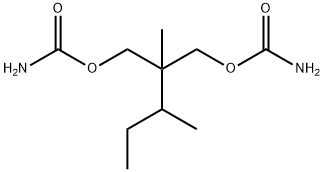
The following example illustrates the preparation of 2-methyl-2-sec-butyl-1,3- propanediol:
92 g of diethyl-sec-butyl methyl malonate were reduced in the usual manner using 22.8 g of lithium aluminum hydride in a suitable volume of anhydrous ethyl ether. The mixture was treated with 10% sulfuric acid and the ether soluble components extracted. The ether solution was dried, using a suitable drying agent, and the residue obtained by the removal of the ether was purified by distilling under reduced pressure. This material was further purified by redistillation. Approximately 46 g of 2-methyl-2-sec-butyl-1,3-propanediol were obtained as a clear colorless liquid, boiling point 92°C to 97°C (0.1 mm pressure).
The following example describes the preparation of 2-methyl-2-sec-butyl-1,3- propanediol dicarbamate using the urethane exchange method:
14.6 g of 2-methyl-2-sec-butyl-1,3-propanediol and 18.7 g ethyl urethane are dissolved in about 100 ml anhydrous toluene. 3 g of aluminum isopropylate are added and the mixture distilled to remove the ethyl alcohol formed in the condensation of ethyl urethane and the diol. The alcohol distills in the form of an azeotrope with toluene. Distillation is continued until the theoretical quantity of ethanol has been removed. The toluene is distilled from the mixture under reduced pressure and the residue dissolved in hot aqueous isopropanol solution. The hot solution is filtered and allowed to cool, whereupon approximately 14 g of product separates. The purified product represents a yield of about 60% of theoretical and melts at 77°C to 79°C.