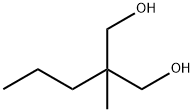
Synthesis
2-Methyl-2-pentenal was hydrogenated at 100°C and 1.3-1.5MPa with a yield of 80%. After obtaining 2-methylpentenal, it was added to 36% formaldehyde, and the reaction was carried out at room temperature. Sodium hydroxide solution was added dropwise. The reaction was exothermic, and the temperature was naturally raised to 70 °C, then heated to 90 °C, added for 1 h, cooled to 60 °C and left to separate layers. The upper layer was washed with water, the washings were combined with the lower layer, and extracted with benzene. The extract and the upper layer were combined, and benzene was recovered by distillation, followed by distillation under reduced pressure to collect a fraction at 160°C (14.7kPa), which was 2-methyl-2-propyl-1,3-propanediol,yield 93%.
Description
2-Methyl-2-propyl-1,3-propanediol is produced either by the salt-free procedure based on aldol addition followed by hydrogenation or by the classical combination of hydroxymethylation and Cannizzaro-type disproportionation with formaldehyde. The production costs are considerably higher than for neopentyl glycol, because the synthesis of 2-methylpentanal from propanal requires two additional steps: aldol condensation and hydrogenation.
Chemical Properties
Whgite Crystalline Solid
Uses
An intermediate in the synthesis of Carisoprodol.
Uses
2-Methyl-2-propyl-1,3-propanediol is mainly used in pharmaceutical applications such as production of dicarbamates. Meprobamate (2-methyl-2-propyl-1,3-propanediol dicarbamate), is used as an anxiolytic drug (tranquilizer). Carisoprodol (N-isopropyl-2-methyl-2-propyl-1,3-propanediol dicarbamate), possesses strong centrally-acting muscle relaxant activity.