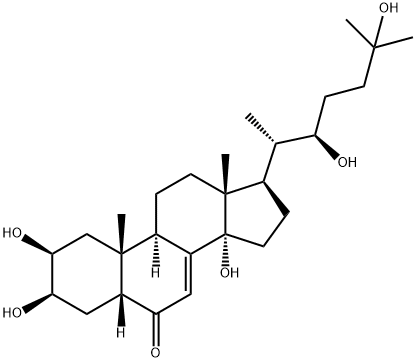
Ecdysone
- Product NameEcdysone
- CAS3604-87-3
- CBNumberCB8113524
- MFC27H44O6
- MW464.63
- EINECS222-760-4
- MDL NumberMFCD00042683
- MOL File3604-87-3.mol
Chemical Properties
| Melting point | 242°C |
| alpha | 20578 +62° |
| Boiling point | 488.1°C (rough estimate) |
| Density | 1.0493 (rough estimate) |
| refractive index | 1.4482 (estimate) |
| storage temp. | 2-8°C |
| solubility | Methanol (Very Slightly, Heated) |
| form | Solid |
| pka | 14.07±0.70(Predicted) |
| color | White to Off-White |
| optical activity | +5820 (c 0.1, ethanol) |
| Merck | 13,3525 |
| BRN | 2422986 |
| InChIKey | UPEZCKBFRMILAV-JMZLNJERSA-N |
| SMILES | [C@H]1(C[C@@]2([C@](C)(C[C@@H]1O)[C@]1([H])C([C@]3([C@@](CC1)(C)[C@@H]([C@H](C)[C@@H](CCC(C)(C)O)O)CC3)O)=CC2=O)[H])O |
| LogP | 0.870 (est) |
| CAS DataBase Reference | 3604-87-3(CAS DataBase Reference) |
| FDA UNII | RH692X7B7B |


