Chemical Properties
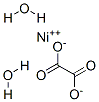
light green powder(s) . Nickel(II) Oxalate Dihydrate [6018-94-6], Ni(COO)2·2 H2O, Mr 182.76, precipitates as a green powder when oxalic acid or alkali-metal oxalate solutions are added to nickel(II) salt solutions. When heated in vacuum, it evolves water at 150 ℃ and decomposes completely at 300 ℃, giving finely divided nickel suitable for use in catalysis or in the production of nickel carbonyl.
Uses
Nickel oxalate is used for the production of nickel catalysts and magnetic materials.
Uses
It is an important raw material and intermediate used in organic Synthesis, pharmaceuticals, agrochemicals and dyestuffs.
Preparation
Nickel(II) oxalate precipitates as NiC2O4?2H2O when oxalic acid is added to nickel
salt solutions. It is isomorphous with the oxalates of magnesium, zinc and iron(II), and has
μ
eff=2.95 BM. When heated in vacuo it decomposes evolving water above 150° and
carbon dioxide above 300° leaving finely divided nickel suitable for catalytic purposes or
reacting to form the carbonyl. The oxalate dissolves in acids with decomposition and in
ammonia with ammine formation.
Production Methods
Nickel Oxalate is produced as a greenish white crystalline dihydrate.