Description
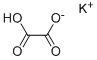
Potassium hydrogen oxalate, also known as potassium bioxalate, is a salt with formula KHC
2O
4 or K
+·HO
2C-CO
2-. It is one of the most common salts of the hydrogenoxalate anion, and can be obtained by reacting potassium hydroxide with oxalic acid in 1:1 mole ratio.
The salt is also known as potassium hydrogen oxalate , acid potassium oxalate, or monobasic potassium oxalate. In older literature, it was also called sorrel salt, sal acetosella ,
Potassium hydrogen oxalate occurs in some plants, notably sorrel. It is a commercial product, used in photography, marble grinding, and to remove ink stains.
Chemical Properties
The anhydrous product is a white, odorless, crystalline solid, hygroscopic and soluble in water (2.5 g/100 g at room temperature). The solutions are basic. Below 50 °C the much less soluble potassium tetraoxalate forms and precipitates out of solution.
The monohydrate KHC
2O
4·H
2O starts losing the water at 100 °C.
The anhydrous salt was found to have remarkable elastic anisotropy, due to its crystal structure that consists of relatively rigid columns of hydrogen-bonded hydrogenoxalate anions, joined into sheets by ionic K–O bonds. .
History
Potassium acid oxalate, KHC2O4, exists as a monohydrate. It is of historical interest because it is the salt of sorrel found in vegetation and the first oxalate isolated.
Uses
Removing ink stains, scouring metals, cleaning
wood, photography, laboratory reagent, mordant.
General Description
Odorless white solid. Sinks in water.
Air & Water Reactions
Hygroscopic. Gives basic solution, below 50°C dissolves in water and reacts to form the much less soluble potassium tetraoxalate, which separates out.
Reactivity Profile
Salts, basic, such as Potassium binoxalate, are generally soluble in water. The resulting solutions contain moderate concentrations of hydroxide ions and have pH's greater than 7.0. They react as bases to neutralize acids. These neutralizations generate heat, but less or far less than is generated by neutralization of the bases in reactivity group 10 (Bases) and the neutralization of amines. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible.
Hazard
Toxic by ingestion.
Health Hazard
Inhalation of dust causes irritation of nose and throat. Ingestion causes burning pain in throat, esophagus, and stomach; exposed areas of mucous membrane turn white; vomiting, severe purging, weak pulse, and cardiovascular collapse; if death is delayed, neuromuscular symptoms develop. Contact with dust irritates eyes and may cause mild irritation of skin.
Flammability and Explosibility
Non flammable
Safety Profile
Moderately toxic to humans by ingestion. Human systemic effects by ingestion and intravenous routes: general anesthetic, somnolence, fluid intake, blood pressure increase or decrease, esophagus changes,nausea or vomiting, and urine volume decrease or anu
Toxicology
Potassium hydrogen oxalate is strongly irritating to eyes, mucoses and gastrointestinal tract. It may cause cardiac failure and death .
Purification Methods
Crystallise it from H2O by dissolving 20g in 100mL H2O at 60o containing 4g of potassium oxalate, filtering and allowing to cool to 25o. The crystals, after washing three or four times with water, are allowed to dry in air. [Beilstein 2 III 1552.]