Description
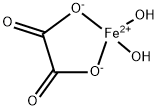
Iron(II) oxalate, FeC204.2H20, is precipitated as yellow crystals from solutions
containing iron(II) and oxalate ions ; in the presence of excess alkali metal oxalate, however,
soluble oxalato complexes M2[Fe(C2O4)2] are formed which can be precipitated by the
addition of alcohol. The oxalate is paramagnetic with μeff= 5·2 B.M. at room temperature.
Chemical Properties
yellow powder
Ferrous oxalate, or iron(II) oxalate, is a chemical compound consisting of one iron(II) ion (Fe2) and one oxalate ion (C2O4(2−)). It has the chemical formula FeC2O4. Iron(II) oxalate is more commonly encountered as the dihydrate, FeC2O4·2H2O, CAS # 6047-25-2. Its crystal structure consists of chains of oxalate-bridged iron atoms, capped by water molecules. When heated, it dehydrates and decomposes into carbon dioxide, carbon monoxide, iron oxides and pyrophoric black iron.