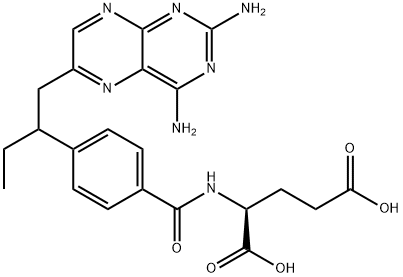
Edatrexate (0.14 g/kg and 0.21 g/kg; i.p.; twice weekly×3 or weekly×3) exerts potent efficacy against advanced metastatic disease in tumor-bearing mice, better than MTX[1].
| Animal Model: | Murine models of advanced metastatic disease (including E0771 mammary adenocarcinoma, T241 fibrosarcoma, Lewis lung carcinoma, B16 melanoma, or C38 colon carcinoma)[2] |
| Dosage: | 0.14 g/kg and 0.21 g/kg |
| Administration: | Intraperitoneal injection; 0.14 g/kg twice weekly for 3 weeks and 0.21 g/kg weekly for 3 weeks; added Ca leucovorin (LCV) 16, 20, 24 h after Edatrexate treatment |
| Result: | Increased survival of tumor-bearing mice.
Showed markedly therapeutic effectiveness against advanced metastatic disease in high-dose regimen with delayed LCV rescue. |