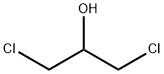
Chemical Properties
There are 4 isomers of dichloropropanols 1,3-
dichloro-2-propanol (96-23-1) and “dichloropropanols”
(26545-73-3) are citations in environmental regulations:
C3H6OCl2 is a colorless viscous liquid with a chloroformlike odor. Slightly soluble in water. 1,3-Dichloro-2-
propanol:
Chemical Properties
Colorless to light yellow liqui
Uses
A labelled chloropropanol which shows toxic effects.
Uses
1,3-Dichloro-2-propanol biotransformation to epichlorohydrin by the whole cells of recombinant Escherichia coli via resin-based in situ product removal has been investigated. It is used in the synthesis of glycerol, in the production of plastics and textiles and in the synthesis of pharmaceuticals. Also used as a solvent and as a cement for celluloid. It is also present in some foods, e.g. soy sauce, soup spices and instant soups, from hydrolization of proteins.
Uses
A chloropropanol which shows toxic effects.
Definition
ChEBI: 1,3-dichloropropan-2-ol is a secondary alcohol that is isopropanol in which one hydrogen of each methyl group is substituted by a chlorine. A liquid at room temperature (melting point -4℃, boiling point 174℃ at 760 mm Hg), it is used as a solvent for hard resins and nitrocellulose. It has a role as a protic solvent and a cross-linking reagent. It is a secondary alcohol and an organochlorine compound.
General Description
Colorless to yellow slightly viscous liquid with an ethereal odor.
Air & Water Reactions
Water soluble.
Reactivity Profile
Sensitive to heat. Incompatible with oxidizers. Also incompatible with strong acids, strong reducing agents, acid chlorides and acid anhydrides.
Fire Hazard
1,3-Dichloro-2-propanol is combustible.
Flammability and Explosibility
Not classified
Safety Profile
Suspected carcinogen.
Poison by ingestion and inhalation.
Moderately toxic by skin contact. Human
mutation data reported. A skin irritant.
Action may be similar to that of carbon
tetrachloride, but more irritating to mucous
membranes. Flammable when exposed to
heat, flame, or oxidizers. To fight fire, use
alcohol foam, dry chemical, fog, mist, or
spray. Dangerous; when heated to
decomposition it emits highly toxic fumes of
Cl and phosgene.
Potential Exposure
It is used as a solvent for hard resins
and nitrocellulose; in the manufacture of photographic
chemicals and lacquer; as a cement for celluloid; and as a
binder of water colors. It occurs in effluents from glycerol
and halohydrin production plants.
Shipping
UN2750 1,3-Dichloropropanol-2, Hazard Class:
6.1; Labels: 6.1-Poisonous materials
Incompatibilities
Incompatible with oxidizers (chlorates,
nitrates, peroxides, permanganates, perchlorates, chlorine,
bromine, fluorine, etc.); contact may cause fires orexplosions. Keep away from alkaline materials, strong
acids, acid anhydrides, strong bases
Toxics Screening Level
The current initial threshold screening level (ITSL) for 1,3-dichloro-2-propanol is 3 μg/m3
based on an annual averaging time.