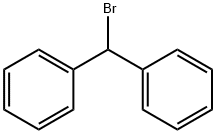
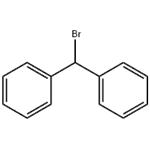
Uses
Benzhydryl Bromide is used as a reagent in the synthesis of O-(triazolyl)methyl carbamates as a novel and potent class of fatty acid amide hydrolase (FAAH) inhibitors.
Chemical Properties
ORANGE-RED TO ORANGE-BROWN LOW MELTING SOLID
Uses
Benzhydryl bromide is a halogenated building block. Benzhydryl group was preferred to the commoner benzyl to protect 2-nitrophenol in the Bartoli (vinyl Grignard) synthesis of 7-hydroxyindole. Protection was by reaction with the phenol in the presence of potassium carbonate in acetone; deprotection of the indole was by hydrogenolysis.
General Description
Bromodiphenylmethane is a white crystalline solid. Melting point 113°F. A lachrymator. In the presence of moisture corrosive to tissue and most metals.
Air & Water Reactions
Insoluble in cold water. Decomposed by hot water to give corrosive hydrobromic acid.
Reactivity Profile
Halogenated aliphatic compounds are moderately or very reactive. Reactivity generally decreases with increased degree of substitution of halogen for hydrogen atoms. Materials in this group are incompatible with strong oxidizing and reducing agents. Also, they are incompatible with many amines, nitrides, azo/diazo compounds, alkali metals, and epoxides.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Combustible material: may burn but does not ignite readily. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form.
Safety Profile
A corrosive, irritating
liquid, When heated to decomposition it
emits toxic fumes of Br-. See also