Chemical Properties
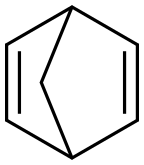
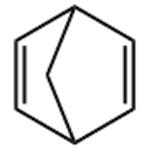
2,5-Norbornadiene, also known as dicycloheptadiene, is a clear colorless to slightly yellow liquid that is soluble in petroleum ether and insoluble in water. Commercially available goods usually contain 500 ppm of 2,6-di-tert-butyl-p-methylphenol (BHT) as a stabilizer. Analyzing the structural characteristics of NBD, it can be found that it has good reaction properties and can synthesize a variety of organic compounds through addition or substitution reactions at the double bond.
Uses
2,5-Norbornadiene is used as an intermediate in prostaglandin synthesis and shows anti-ethylene effect on plants. It acts as a starting material for the synthesis of diamantane and as an acetylene transfer agent for instance, in reaction with 3,6-di-2-pyridyl-1,2,4,5-tetrazine. It is also a useful dienophile in Diels-Alder reactions.
Uses
2,5-Norbornadiene is industrially available
as a chemical intermediate. It was not intended
for use in perfumes, and it is only occasionally
used as such. It serves mainly as a starting
material for the manufacture of industrial
chemicals, etc.
2,5-Norbornadiene has occasionally been used in
Pine needle fragrances to improve the diffusiveness of the fragrance, and to lend initial
power and short life to the fragrances which
are intended for "short-effect" purposes, e.g.
dishwashing detergent fragrances, etc.
It is also used as a masking agent when a
volatile and powerful material is needed at
low cost.
Preparation
2,5-Norbornadiene is of interest as a metal-binding ligand, whose complexes are useful for homogeneous catalysis. Norbornadiene can be formed by a Diels-Alder reaction between cyclopentadiene and acetylene.
General Description
2,5-norbornadiene, stabilized appears as a colorless liquid. Insoluble in water and less dense than water. Contact may irritate skin, eyes and mucous membranes. May be toxic by ingestion, inhalation and skin absorption. When heated will polymerize and at decomposition emits acrid smoke and irritating fumes.
Air & Water Reactions
Highly flammable. Insoluble in water.
Reactivity Profile
2,5-NORBORNADIENE may react vigorously with strong oxidizing agents. May react exothermically with reducing agents to release hydrogen gas. In the presence of various catalysts (such as acids) or initiators, may undergo exothermic addition polymerization reactions. Violent explosions at low temperatures in ammonia synthesis gas units have been traced to the addition products of dienes and nitrogen dioxide [Bretherick, 5th Ed., 1995].
Health Hazard
Inhalation or contact with material may irritate or burn skin and eyes. Fire may produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.
Safety Profile
Poison by intravenous
route. Moderately toxic by ingestion and
intraperitoneal routes. Mildly toxic by
inhalation. A flammable liquid. When heated
to decomposition it emits acrid smoke and
irritating fumes.
Purification Methods
Purify the diene by distillation from activated alumina [Landis & Halpern J Am Chem Soc 109 1746 1987]. [Beilstein 5 IV 879.]