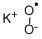Chemical Properties
light yellow powder or chunks
Uses
Potassium oxide is used as a carbon dioxide scrubber, water dehumidifier and oxygen generator. It finds application in rebreathers for fighting with fire and mine rescue work. It is also used in spacecraft, submarines and spacesuit life support systems.
Uses
Reagent and intermediate.
One use of potassium superoxide,KO2, is for generating oxygen. It has the ability to absorb carbon dioxide, while giving out oxygen at the same time:
4KO2(s)+ 2CO2(g)--->2K2CO3(s)+ 3O2(g)
This property has been made use of in breathing equipment,e.g.for mountaineers, in submarines and in spacecraft.
General Description
A yellowish to white solid. Melting point 948°F. Mixtures with combustible material readily ignite by friction, heat, or contact with moisture. Prolonged exposure to fire or heat may cause vigorous decomposition of the material and rupturing of the container.
Air & Water Reactions
Reacts explosively with water [Mellor 2, Supp. 3: 1631. 1963].
Reactivity Profile
Potassium superoxide is a powerful oxidizer. Forms on the surface of potassium metal, solid or molten, that is exposed to the air. Attempts to extinguish burning potassium with powdered graphite has resulted in violent explosions [Chem. Abstr. 63:424. 1965]. Highly oxidized potassium metal was dropped into a dish of ethyl alcohol, an immediate explosion shattered the dish. Potassium superoxide was considered the cause of the reaction [Health and Safety Inf. 251. 1967]. Potassium superoxide should not be added to pure organic materials (hydrocarbons), as ignition and violent explosion may occur. Oxidation of arsenic, antimony, copper, potassium, tin, or zinc proceeds with incandescence, [Mellor, 1941, Vol. 2, 493]. Interaction between the superoxide and diselenium dichloride is violent, [Mellor, 1947, Vol. 10, 897].
Hazard
Corrosive to tissue.
Health Hazard
TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Fire may produce irritating and/or toxic gases. Toxic fumes or dust may accumulate in confined areas (basement, tanks, hopper/tank cars, etc.). Runoff from fire control or dilution water may cause pollution.
Fire Hazard
May explode from friction, heat or contamination. These substances will accelerate burning when involved in a fire. May ignite combustibles (wood, paper, oil, clothing, etc.). Some will react explosively with hydrocarbons (fuels). Containers may explode when heated. Runoff may create fire or explosion hazard.
Safety Profile
Explosive reaction
when heated with carbon, 2-aminophenol +
tetrahydrofuran (at 65°C). Forms a friction-
sensitive explosive mixture with
hydrocarbons. Violent reaction with
lselenium dichloride, ethanol, potassium-
sodium alloy. May ignite on contact with
organic compounds. Incandescent reaction
with metals (e.g., arsenic, antimony, copper,
potassium, tin, and zinc). When heated to
decomposition it emits toxic fumes of K2O.
See also PEROXIDES.