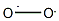Description
A compound characterized by the presence in its structure of the O2− ion. The O2− ion has an odd number of electrons (17) and as a result all superoxide compounds are paramagnetic. At room temperature they have a yellowish color. At low temperature many of them undergo reversible phase transitions accompanied by a color change to white. The stable superoxides are
sodium superoxide NaO2
potassium superoxide KO2
rubidium superoxide RbO2
cesium superoxide CsO2
calcium superoxide Ca(O2)2
strontium superoxide Sr(O2)2
barium superoxide Ba(O2)2
tetramethylammonium superoxide (CH3)4NO2
In these compounds each oxygen atom has an oxidation number of −1/2 instead of −2, as a normal oxide.
Description
A product of the univalent reduction of O2
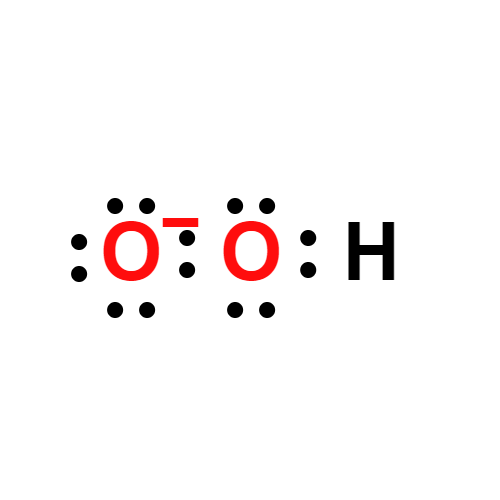
or the univalent oxidation of H2O2. The superoxide radical (O22 ) or its corresponding acid (perhydroxyl radical, HO2; pKa 5 4.8)
is generated both enzymatically and nonenzymatically in biological systems. Superoxide can also be produced by cathodic
reduction of O2 in an aprotic solvent, as well as by ultrasonication, pulse radiolysis, or photolysis of water. By virtue of a relatively negative dioxygen/superoxide couple (Eo0
5 20.31 V)
superoxide is a stronger reductant than it is an oxidant (i.e., it
readily reduces oxidized cytochrome C). Detection methods
include optical spectroscopy, electron spin resonance, mass spectrometry, and detection of its presence based on its ability to
reduce readily measurable oxidants such as cytochrome c or tetrazolium salts. Due to its reductive ability as well as the spontaneous disproportionation that proceeds with a rate constant of
about 105 M21 sec21 (O22 1 O22 1 2H1-H2O2 1 O2), the
steady-state concentrations of the oxyradicals are usually low at
physiological conditions.
Definition
An inorganic compound containing the O
2– ion.
Definition
superoxides: group of inorganiccompounds that contain the O
2– ion.They are formed in significant quantitiesonly for sodium, potassium, rubidium,and caesium. They are verypowerful oxidizing agents and reactvigorously with water to give oxygengas and OH– ions. The superoxide ionhas an unpaired electron and is paramagneticand coloured (orange).