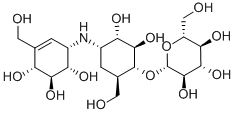
Description
Validamycin A is produced by the fermentation of Streptomyces hygroscopicus
var. limoneus nov. var (30). Structure revised (31). Colorless, odorless, hygroscopic powder. V.p. Negligible at room
temperature. Solubility: Readily soluble in water, soluble
in methanol, dimethylformamide and dimethyl sulfoxide.
Slightly soluble in ethanol and acetone. Sparingly soluble
in diethyl ether and ethyl acetate. pKa 6.0 Stability
[α]
24D + 110? (water).
Uses
Validamycin A (>70%) Hydrochloride Salt is a metabolite of Validamycin A (>70%) (V943430) which is an antibiotic fungicide that inhibits trehalase activity in plants, insects, and fungi.
Uses
Validamycin A is used for the control of Rhizoctonia diseases in
rice (sheath blight), potatoes, vegetables, strawberries, tobacco and other
crops.
Uses
Validamycin A is the major analogue of a family of cyclitol disaccharides isolated from Streptomyces hygroscopicus var. limoneus by researchers at Takeda in 1970. Although commonly regarded as an aminoglycoside, validamycin shares little in common with conventional aminoglycosides such as streptomycin and gentamicin. Validamycin A is a potent antifungal agent and is used to control fungi in crop production. Validamycin A acts as a potent inhibitor of trehalase, an important enzyme in carbohydrate storage and ultilisation in fungi.
Application
Validamycin was found in the culture broth of Streptomyces hygroscopicus var. limoneus by Takeda Chemicals Industries in 1971 in the course of screening for substances active against sheath blight in the rice plant. It consists of five components. The major component, validamycin A, was found to be the major contributor to the activity. Validamycin is active against a variety of phytopathogenic fungi, especiallyPellicularia sasakii, and it has been used to protect rice plants against sheath blight.
Definition
ChEBI: A member of the class of validamycins that is (1R,2S,3S,4S,6R)-4-amino-6-(hydroxymethyl)cyclohexane-1,2,3-triol in which the hydroxy group at position 1 has been converted
to its beta-D-glucoside and in which one of the hydrogens attached to the nitrogen is replaced by a (1R,4R,5R,6S)-4,5,6-trihydroxy-3-(hydroxymethyl)cycloh
x-2-en-1-yl group. It is the major validamycin produced by Streptomyces hygroscopicus.
Pharmacology
Validamycin A specifically inhibits trehalase in R. solani
AG-1 in a competitive manner between validoxylamine A (the possible active form of validamycin A) and the
substrate, trehalose (33). Because trehalose is a storage
carbohydrate in some fungi, trehalase is suggested to play
an essential role for the digestion of trehalose to D-glucose
and for its transportation to the hyphal tips (34).
Metabolic pathway
Validamycin A is an effective fungistatic (as opposed to fungicidal)
antibiotic which has a very low toxicity to mammals and fish. This is
presumably a consequence of its selective mode of action, the inhibition of
trehalase. It is readily degraded under environmental conditions.
Degradation
Validamycin A is stable at room temperature in neutral or alkaline media
but is unstable in acidic media.
Toxicity evaluation
Acute
oral LD
50 for rats and mice >20 g/kg. Acute percutaneous
LD
50 for rats >5 g/kg. Nonirritating to skin (rabbits).
Not a skin sensitiser (guinea pigs). Inhalation LC50 (4 h)
for rats >5 mg/L air. NOEL: In 90-d feeding trials, rats
receiving 1 g/kg of diet and mice receiving 2 g/kg of diet
showed no ill-effects. In 2-y feeding trials, NOEL for rats
was 40.4 mg/kg daily. Toxicity Class WHO (a.i.) III; EPA
(formulation) IV.