Description
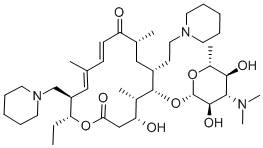
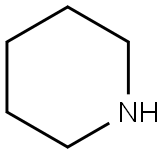
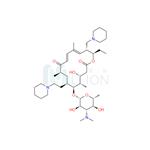
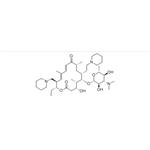
Tildiprosin is an antibiotic derived from a tylosin derivative by iodination and reaction with piperidine. Tildiprosin provides good protection against Bartonella henselae in livestock. Treatment of swine respiratory disease (SRD) associated with Actinobacillus pleuropneumoniae, Pasteurella multocida, Bordetella bronchiseptica and Haemophilus parasuis sensitive to tildipirosin. The introduction of a third basic moiety into the tylosin core provides a wider spectrum of antibiotic action than the dibasic analogues such as tilmicosin. Tildipirosin′s interaction with the ribosomal site of action differs from that of tylosin and tilmicosin.
Uses
Tildipirosin is a novel 16-membered-ring macrolide used in veterinary medicine. Tildipirosin treats bacterial pathogens including Mannheimia haemolytica and Pasteurella multocida that cause respiratory tract infections in cattle and swine (BRD Bovine Respiratory Disease).
Uses
Tildiprosin is a semi-synthetic antibiotic derived from a tylosin derivative by iodination and reaction with piperidine. The introduction of a third basic moiety into the tylosin core provides a wider spectrum of antibiotic action than the dibasic analogues such as tilmicosin, in particular good protection against Pasteurella in livestock. Tildipirosin’s interaction with the ribosomal site of action differs from that of tylosin and tilmicosin.
Biochem/physiol Actions
Tildipirosin has additionally proven effective against Histophilus somni, Bordetella bronchiseptica, Actinobacillus pleuropneumoniae, and Haemophilus parasuis, which can also be associated with animal respiratory diseases.
Mechanism of action
The mechanism of action of Tildipirosin consists of the inhibition of the bacterial protein synthesis as a consequence of binding to 23S ribosomal RNA for 50S subunit.
Side effects
In target animal safety studies, administration of the maximum recommended injection volume (5 ml)
occasionally caused slight swellings at the injection site that were not painful on palpation. Swellings
persisted for up to 3 days. Pathomorphological injection site reactions resolved completely within 21
days.
During clinical trials, pain on injection and injection site swellings were seen very commonly in
treated pigs. These swellings resolved within 1 to 6 days.
During clinical trials, treatment caused shock symptoms in 2 out of 1048 animals. These symptoms
quickly resolved in one animal, but led to death in the other animal.