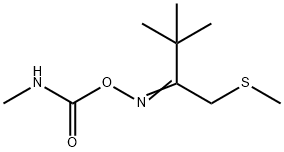
Chemical Properties
Thiofanox is a colorless solid with a pungent odor.
Uses
Thiofanox is a systemic soil insecticide used for the control of
aphids, mites, thrips, plant bugs, leafhoppers and beetles in/on sugar
beet and potatoes.
General Description
THIOFANOX is a colorless solid with a pungent odor. Used as a systemic insecticide and acaricide.
Air & Water Reactions
Thio and dithiocarbamates slowly decompose in aqueous solution to form carbon disulfide and methylamine or other amines. Such decompositions are accelerated by acids.
Reactivity Profile
THIOFANOX is a thiocarbamate. Flammable gases are generated by the combination of thiocarbamates and dithiocarbamates with aldehydes, nitrides, and hydrides. Thiocarbamates and dithiocarbamates are incompatible with acids, peroxides, and acid halides.
Health Hazard
THIOFANOX is a carbamate pesticide. Carbamate pesticides are moderately to highly toxic. It is a cholinesterase inhibitor.
Fire Hazard
(Non-Specific -- Carbamate Pesticide, Solid, n.o.s.) Container may explode in heat of fire. When heated to decomposition, THIOFANOX emits very toxic fumes of nitrogen and sulfur oxides. Stable at normal storage temperature; reasonably stable to hydrolysis at less than 86F at pH 5-9.
Pharmacology
The insecticidal carbamates are cholinergic. Poisoned
insects and animals exhibit violent convulsions and
other neuromuscular disturbances. These insecticides
carbamylate acetylcholinesterase and may have a direct
action on acetylcholine receptors. The mechanism of interaction
with acetylcholinesterase is analogous to the normal
three-step hydrolysis of acetylcholine. However, the third
reaction step is much slower for the carbamylated enzyme
than for the acetylated one. The importance of structural
complementarity of the insecticidal carbamates to the
active site of acetylcholinesterase is demonstrated by the
pronounced difference in activities of D-2-(sec-butylphenyl)
methylcarbamate and L-2-(sec-butylphenyl) methylcarbamate
(the L isomer is five times more toxic) and of the 2-,
3-, and 4-substituted phenylmethylcarbamates, where the
4-isomers are virtually inactive.
Detoxification of carbamate insecticides occurs in vivo
through microsomal hydroxylation, N-demethylation of
carbamyl nitrogen, side chain oxidation, and ring hydroxylation.
Methylenedioxyphenyl synergists prevent oxidation
Potential Exposure
A potential danger to those involved in the manufacture, formulation and application of this thiocarbamate systemic insecticide and acaricide.
Metabolic pathway
The hydrolytic degradation and metabolism of thiofanox in soils, plants
and animals follow a common pathway. Oxidation of the S-methyl moiety
is the primary reaction to yield the thiofanox sulfoxide and sulfone.
Hydrolysis of the carbamate linkage to yield the oximes is only a minor
pathway for thiofanox and its oxidation products (Scheme 1).
Shipping
UN2757 Carbamate pesticides, solid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials. UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Degradation
Thiofanox (1) is stable to hydrolysis at acidic and neutral pH levels, but
readily degrades in alkaline solution (>pH 10). The major products
resulted from the oxidation of the S-methyl moiety to yield thiofanox
sulfoxide (2) and thiofanox sulfone (3). Hydrolysis of thiofanox and its
oxidation products to the corresponding oximes (4, 5 and 6), and the
further oxidation of thiofanox sulfone oxime (6) to the ketone (7) were
minor reactions (Chin et al., 1976).
Incompatibilities
Thiocarbamate esters are combustible. They react violently with powerful oxidizers such as calcium hypochlorite. Poisonous gases are generated by the thermal decomposition of thiocarbamate compounds, including carbon disulfide, oxides of sulfur, oxides of nitrogen, hydrogen sulfide, ammonia, and methylamine. Many materials in this group slowly decompose in aqueous solution to form carbon disulfide and methylamine or other amines. Such decompositions are accelerated by acids. Flammable gases are generated by the combination of
Waste Disposal
In accordance with 40CFR 165 recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.