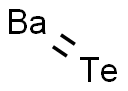Description
Barium telluride has the molecular formula of BaTe
and the molecular weight of 264.9270 g/mol. It can be
prepared by heating the elements together in an inert
atmosphere:
Ba+ Te+ heat ? BaTe
Te melts at 450°C and Ba melts at 728°C. A heating
temperature of 750 Cis sufficient to cause complete reaction.
BaTe powder can also be prepared by reaction of
stoichiometric quantities of Ba and Te in a pure graphite
crucible in vacuum at 480°C during 48 h. Its molecular
weight is 216.31 g/mol. The heat of formation, △H
0 at
that temperature is 9.29 kJ/mol. Barium telluride can also be prepared by direct reduction of barium tellurate.
Uses
Barium telluride has been sadly overlooked in
industry but not as a research subject in the literature.
According to early sources, BaTe ought not to exist but
a few workers have found methods to prepare it.However, the physical data such as melting point for
this compound have not been available. It is, however,
readily available commercially.