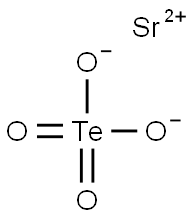Description
Strontium tellurate has the molecular formula of
SrTeO4 and the molecular weight of 279.216 grams per
mole. Its CAS number is 15852-10-5. It can be prepared
by the solid-state reaction of TeO3 and SrO:
SrO + TeO3+ heat ? SrTeO4
TeO3 melts at 430°C and a temperature of 600°C
serves to drive the reaction to completion. This is the
tetragonal meta-tellurate composition. It can also be formed in the same manner by solid-state reaction of
telluric acid:
SrO(solid) + H2TeO4(solid) ? SrTeO4(solid) +H2O(gas)
This is also tetragonal in structure, with the meta-tellurate
configuration. Of the two possible configurations,
the meta-tellurate is said to be the most stable.
Telluric acid is soluble in water and produces
Te(OH)4O
2-
anions in solutions. If strontium chloride
is added, the product is a tetrahydrate:
SrCl2(aq) +H2Te(OH)4(aq)? SrTeO2(OH)4