Manufacturing Process
3.7 parts of 2-(4-chlorophenyl)-4-cyanomethylthiazole and 35 parts of 6 N
hydrochloric acid are heated under reflux for 2 hours. The solution is cooled
by the addition of ice and made alkaline to pH 8 by the addition of 30%
aqueous ammonia. The mixture is filtered to remove trace impurities, and an
excess of 40% sodium hydroxide solution is then added to the filtrate to cause
precipitation of a sodium salt, which is collected by filtration and crystallised
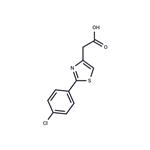
from water. There is thus obtained sodium 2-(4-chlorophenyl)thiazol-4-
ylacetate, M.P. 13°C (decomposition).
This sodium salt is dissolved in hot water, and the solution is brought to pH 4
by the addition of acetic acid, which causes the precipitation of 2-(4-
chlorophenyl)thiazol-4-ylacetic acid. This is collected by filtration, washed with
water, and dried in vacuo over phosphorus pentoxide. It has an M.P. 155-
156°C (from ethyl acetate).
![2-[2-(4-CHLOROPHENYL)-1,3-THIAZOL-4-YL]ACETIC ACID Structure](https://www.chemicalbook.com/CAS/GIF/17969-20-9.gif)