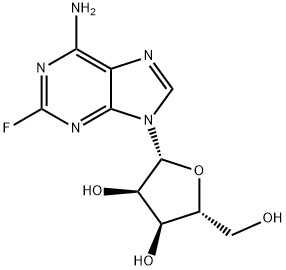
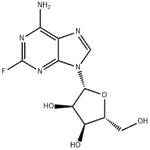
Description
2-Fluoroadenosine (F-Ado) was developed at Southern Research Institute in 1957 as a potential anticancer drug. 2-Fluoroadenosine is not deaminated by adenosine deaminase but metabolized to triphosphate as shown in vitro. The drug was also shown to be a potent inhibitor of lymphocyte-mediated cytolysis.
Uses
2-Fluoroadenosine is a fluorinated analog of Adenoside nucleotide. It is used as an intermediate for the drug fludarabine. Fludarabine is a purine analogue and antineoplastic agent. It is a chemotherapy medication used in the treatment of leukemia and lymphoma.
Definition
ChEBI: 2-fluoroadenosine is a member of adenosines and an organofluorine compound.
Biological Functions
2-Fluoroadenosine (F-Ado) was synthesized by Montgomery and his co-workers. This compound has been found to suppress the growth of H.Ep. No. 2 cells growing in culture. Bennett and Smithers have shown that in the same cells, the synthesis of 5-phosphoribosylamine was markedly inhibited by both 2- fluoroadenine and F-Ado. The compound was found to inhibit the incorporation of variously labeled precursors into ribonucleic acid in whole Ehrlich ascites cells. Upon incubation with ascites cells, the nucleoside was readily converted to the 5’-mono-, 5’-di-, and 5’-triphosphates.In addition, striking potentiation of the inhibition of human platelet aggregation by combinations of forskolin with PGE 1 or F-Ado [1-2].
Synthesis
A novel method for the introduction of fluorine in the purine 2-position is described and employed in the synthesis of a potential antimycobacterial compound. Also 2-fluoroadenosine has been synthesized for the first time from adenosine with perbenzoylated 2-nitroadenosine as a key intermediate. Mild reaction conditions are employed and few synthetic steps are required. The novel synthesis of fluoroadenosine can be regarded as a formal synthesis of the antileukemic drug fludarabine phosphate.
[1] MORTEN BR?NDVANG L. G. A Novel Method for the Introduction of Fluorine into the Purine 2-Position: Synthesis of 2-Fluoroadenosine and a Formal Synthesis of the Antileukemic Drug Fludarabine[J]. Synthesis-Stuttgart, 2006, 87 1: 2993-2995. DOI:
10.1055/S-2006-942544.
References
[1] H T Shigeura. “Metabolism of 2-fluoroadenosine by Ehrlich ascites cells.” Archives of biochemistry and biophysics 111 3 (1965): 713–9.
[2] Kailash C. Agarwal, Robert E. Parks Jr. “Synergistic inhibition of platelet aggregation by forskolin plus PGE1 or 2-fluoroadenosine: Effects 2′,5′-dideoxyadenosine and 5′-methylthioadenosine.” Biochemical pharmacology 31 22 (1982): Pages 3713-3716.