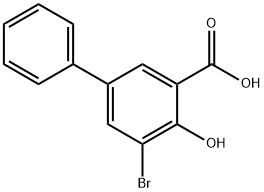
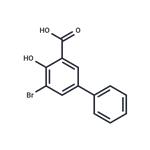
Description
The aldo-
keto reductase (AKR) enzymes constitute a family of related NADPH-
dependent oxidoreductases. The
1C subfamily (AKR1C) includes four human hydroxysteroid dehydrogenases, with AKR1C1 being a 20α-
HSD and the other three being 3α-
HSDs. AKR1C1 metabolizes progesterone to an inactive progestin, 20α-
hydroxy progesterone. In addition, AKR1C1 actions have been implicated in cancer and in the processing of neuroactive steroids involved in brain function. 3-
bromo-
5-
phenyl Salicylic acid selectively inhibits AKR1C1 (K
i = 4 nM) over AKR1C2 (K
i = 87 nM), AKR1C3 (K
i = 4.2 μM), and AKR1C4 (K
i = 18.2 μM). Moreover, it potently inhibits the metabolism of progesterone by bovine aortic endothelial cells overexpressing AKR1C1 (IC
50 = 460 nM).