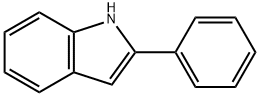
Chemical Properties
off-white to beige or slightly green powder
Uses
Phenylindole is a stabilizer in PVC-plastic products (α-phenylindole).
Uses
Reactant for preparation of: • ;Oxopyrrolidine analogs via iodine-catalyzed Markovnikov addition1• ;Tryptophan dioxygenase inhibitors pyridyl-ethenyl-indoles as potential anticancer immunomodulators2• ;Organic light emitting diodes (OLEDs)3• ;Anti inflammatory agents4• ;Potential cyclooxygenase-1 (COX-1)/cyclooxygenase-2 (COX-2) inhibitors5• ;Agents for cancer and malaria therapy6• ;Antifungal and antibacterial agents7• ;Fluorescent probes8Reactant in:• ;Difluorohydroxylation reactions†• ;Mannich-type reactions†
Uses
Reactant for preparation of:
- Oxopyrrolidine analogs via iodine-catalyzed Markovnikov addition
- Tryptophan dioxygenase inhibitors pyridyl-ethenyl-indoles as potential anticancer immunomodulators
- Organic light emitting diodes (OLEDs)
- Anti inflammatory agents
- Potential cyclooxygenase-1 (COX-1)/cyclooxygenase-2 (COX-2) inhibitors
- Agents for cancer and malaria therapy
- Antifungal and antibacterial agents
- Fluorescent probes
Reactant in:
- Difluorohydroxylation reactions
- Mannich-type reactions
Preparation
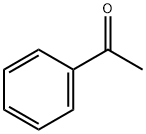
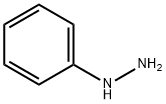
2-phenylindole is prepared by condensing phenylhydrazine and acetophenone, and then closing the ring in the presence of zinc chloride. or it can be prepared by heating bromoacetophenone with excess aniline, or heating N-(2-methylphenyl)benzamide and sodium ethoxide at 360-380℃ in isolation from air.
Definition
ChEBI: 2-phenyl-1H-indole is a phenylindole.