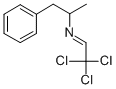
Manufacturing Process
Molar equivalents of d-amphetamine base and chloral hydrate were dissolved in benzene and the solution was heated to distill off benzene and water,
azeotropically. Two molar equivalents of water were separated in this manner.
The remainder of the benzene was distilled off, leaving a liquid residue which
was then fractionally distilled to yield the clear, colorless liquid condensation
product, N-[2-(1-phenylpropyl)]-2,2,2-trichloroethylidenimine, which boiled at
95°C/0.5 mm and had an index of refraction, nD
20 of 1.530 and a specific
optical rotation of +49.9(+/-)0.3° [in dioxane].
Following the above procedure but using dl-amphetamine, the DL-form of N-
[2-(1-phenyl-propyl)]-2,2,2-trichloroethylidemmine was also prepared. It had
the same boiling point and index of refraction as the (-)-form above but its
specific optical rotation was zero.