Chemical Properties
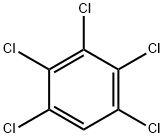
Pentachlorobenzene is a colorless crystalline
solid. Pleasant aroma.
Uses
Agrochemical researc
Uses
It was used to prepare tetrachlorobenzene by photolysis.
Definition
ChEBI: A member of the class of pentachlorobenzenes that is benzene in which five of the hydrogens are replaced by chlorines. Now classed as a persistent organic pollutant under the Stockholm Convention.
Synthesis Reference(s)
The Journal of Organic Chemistry, 38, p. 2928, 1973
DOI: 10.1021/jo00957a002
General Description
White crystals.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
PENTACHLOROBENZENE is relatively unreactive. May be incompatible with strong oxidizing and reducing agents. Also may be incompatible with many amines, nitrides, azo/diazo compounds, alkali metals, and epoxides.
Fire Hazard
Flash point data for PENTACHLOROBENZENE are not available but PENTACHLOROBENZENE is probably non-flammable.
Safety Profile
Moderately toxic by ingestion. An experimental teratogen. When heated to decomposition it emits toxic fumes of Cl-. See also CHLORINATED HYDROCARBONS, AROMATIC
Potential Exposure
Pentachlorobenzene is used primarily
as a precursor in the synthesis of the fungicide pentachloronitrobenzene, and as a flame retardant. Drug/
Therapeutic Agent; Fungicide; bactericide; wood preservative;
industrial Insecticides.
Source
Pentachlorobenzene may enter the environment from leaking dielectric fluids containing
this compound. Pentachlorobenzene may be present as an undesirable by-product in the chemical
manufacture of hexachlorobenzene, pentachloronitrobenzene, tetrachloroenzenes, tetrachloroethylene,
trichloroethylene, and 1,2-dichloroethane (U.S. EPA, 1980).
Environmental Fate
Biological. In activated sludge, <0.1% mineralized to carbon dioxide after 5 days
(Freitag et al., 1985). From the first-order biotic and abiotic rate constants of pentachlorobenzene in estuarine water and sediment/water systems, the estimated biodegradation
half-lives were 4.6–6.5 and 6.0–7.6 days, respectively (Walker et al., 1988)
Photolytic. UV irradiation (λ = 2537 ?) of pentachlorobenzene in a hexane solution
for 3 hours produced a 50% yield of 1,2,4,5-tetrachlorobenzene and a 13% yield of 1,2,3,5-
tetrachlorobenzene (Crosby and Hamadmad, 1971). Irradiation (λ ≥285 nm)
A carbon dioxide yield of 2.0% was achieved when pentachlorobenzene adsorbed on
silica gel was irradiated with light (λ >290 nm) for 17 hours (Freitag et al., 1985).
The experimental first-order decay rate for pentachlorobenzene in an aqueous solution
containing a nonionic surfactant micelle (Brij 58, a polyoxyethylene cetyl ether) and
illuminated by a photoreactor equipped with 253.7-nm monochromatic UV lamps, is 1.47
× 10–2/sec. The corresponding half-life is 47 seconds. Photoproducts reported include, all
tetra-, tri- and dichlorobenzenes, chlorobenzene, benzene, phenol, hydrogen and chloride
ions (Chu and Jafvert, 1994)
Shipping
UN3077 Environmentally hazardous substances,
solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous
hazardous material, Technical Name Required.
Incompatibilities
Polychlorinated hydrocarbons Incompatible
with oxidizers (chlorates, nitrates, peroxides, permanganates,
perchlorates, chlorine, bromine, fluorine, etc.);
contact may cause fires or explosions. Keep away from
alkaline materials, strong bases, strong acids, oxoacids,
epoxides, aluminum, liquid oxygen; potassium, sodium.
Waste Disposal
Consult with environmental
regulatory agencies for guidance on acceptable disposal
practices. Generators of waste containing this contaminant
(≥100 kg/mo) must conform with EPA regulations governing
storage, transportation, treatment, and waste disposal.
Incineration after mixing with another combustible fuel.
Care must be exercised to assure complete combustion to
prevent the formation of phosgene. An acid scrubber is necessary
to remove the halo acids produced.