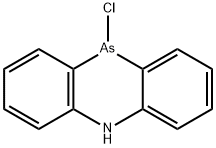
10-chloro-5,10-dihydrophenarsazine
- Product Name10-chloro-5,10-dihydrophenarsazine
- CAS578-94-9
- CBNumberCB5910438
- MFC12H9AsClN
- MW277.58
- EINECS209-433-1
- MOL File578-94-9.mol
Chemical Properties
| Melting point | 195° |
| Boiling point | bp 410° (decompn) |
| Density | 1.65 |
| vapor density | 9.6 |
| form | solid |
| pka | -3.26±0.20(Predicted) |
| color | Light yellow to green granules from xylene or CCl4, or by subl |
| Odor | irritating odor |
| FDA UNII | QI287G628L |
| EPA Substance Registry System | Diphenylaminechloroarsine (578-94-9) |
Safety
| RIDADR | 1698 |
| HazardClass | 6.1(a) |
| PackingGroup | I |
| Hazardous Substances Data | 578-94-9(Hazardous Substances Data) |
| Toxicity | LCLo ihl-hmn: 30 g/m3 SCJUAD 4,33,67 |