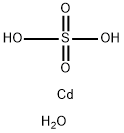
Cadmium sulfate octahydrate
- Product NameCadmium sulfate octahydrate
- CAS7790-84-3
- CBNumberCB5714175
- MFCdH4O5S
- MW228.5
- EINECS616-572-5
- MDL NumberMFCD02253000
- MOL File7790-84-3.mol
- MSDS FileSDS
Chemical Properties
| Melting point | 41 °C |
| Density | 3.08 |
| refractive index | 1.565 |
| storage temp. | Store at +5°C to +30°C. |
| solubility | 1130g/l |
| form | Solid |
| Specific Gravity | 3.08 |
| color | White |
| PH | 3.0-6.0 (50g/l, H2O, 20℃) |
| Odor | Odorless |
| Water Solubility | Highly soluble in water. Partially soluble in methanol and ethyl acetate. Insoluble in ethanol. |
| Merck | 14,1627 |
| Boiling point | >80°C(decomposition) |
| Exposure limits | ACGIH: TWA 0.01 mg/m3; TWA 0.002 mg/m3 NIOSH: IDLH 9 mg/m3 |
| Stability | Stable. |
| InChI | 1S/3Cd.3H2O4S.8H2O/c;;;3*1-5(2,3)4;;;;;;;;/h;;;3*(H2,1,2,3,4);8*1H2/q3*+2;;;;;;;;;;;/p-6 |
| InChIKey | AGHQMAQBLOTWPQ-UHFFFAOYSA-H |
| SMILES | [Cd++].[Cd++].[Cd++].[H]O[H].[H]O[H].[H]O[H].[H]O[H].[H]O[H].[H]O[H].[H]O[H].[H]O[H].[O-]S([O-])(=O)=O.[O-]S([O-])(=O)=O.[O-]S([O-])(=O)=O |
Safety
| Symbol(GHS) |
  
|
|||||||||
| Signal word | Danger | |||||||||
| Hazard statements | H301-H330-H340-H350-H360FD-H372-H410 | |||||||||
| Precautionary statements | P201-P202-P260-P264-P273-P304+P340+P310 | |||||||||
| Hazard Codes | T+,N | |||||||||
| Risk Statements | 45-46-60-61-25-26-48/23/25-50/53 | |||||||||
| Safety Statements | 53-45-60-61 | |||||||||
| RIDADR | UN 2570 6.1/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | EV2850000 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2833 29 20 | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | III | |||||||||
| Storage Class | 6.1A - Combustible acute toxic Cat. 1 and 2 very toxic hazardous materials | |||||||||
| Hazard Classifications | Acute Tox. 2 Inhalation Acute Tox. 3 Oral Aquatic Acute 1 Aquatic Chronic 1 Carc. 1B Muta. 1B Repr. 1B STOT RE 1 | |||||||||
| Toxicity | LD50 orally in Rabbit: 280 mg/kg | |||||||||
| NFPA 704: |
|


