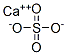Description
Calcium sulfate,CaS04, is a grayish-white dense powder that occurs in nature in both an anhydrous form (anhydrite) and hydrated form(gypsum); it is also the byproduct of many chemical reactions. It has many industrial uses;for example, as a source of sulfur and sulfuric acid,in cements, tiles and plaster,as a soil conditioner, in paints, dyes,and polishes, and as a food additive.
Uses
Calcium Sulfate is most often
used as “gypsum” which is the dihydrate. In the form
of b-anhydrite (the nearly anhydrous form), it is used as a desiccant. It is also used as a coagulant in products
like “tofu”, which is a “bean curd”. When sold as a colorindicating
variant under the name “Drierite ”, it
appears blue or pink due to impregnation with cobalt
chloride, which functions as a moisture indicator.
In its natural state, unrefined calcium sulfate is
a translucent, crystalline white rock. Many forms are
known, including “Alabaster” and “Gypsum”. Selenite,
Satin spar, Desert rose, and Gypsum flower are four
varieties of Gypsum minerals; all four varieties are crystalline.
The four “crystalline” varieties of gypsum are
sometimes grouped together and called selenite. All
have the same chemical formulation, CaSO4·2H2O. All
varieties of gypsum are very soft minerals (hardness: 2
on Mohs scale). This is the most important identifying characteristic of gypsum.
Uses
Pharmaceutic
aid (tablet and capsule diluent).
Uses
Calcium Sulfate is a general additive available as both calcium sul-
fate anhydrous, made by the high-temperature calcining of gypsum
which is then ground and separated, and calcium sulfate dihydrate,
which is made by grinding and separating gypsum containing about
20% water of crystallization. calcium sulfate anhydrous contains
approximately 29% calcium, and calcium sulfate dihydrate contains
approximately 23% calcium. it is used, among other things, as a
filler and baking powder for standardization purposes; a firming
agent in canned potatoes, tomatoes, carrots, lima beans, and pep-
pers; in dough as a source of calcium ions (because the absence of
calcium ions causes bread dough to be soft and sticky and to pro-
duce bread of poor quality); in soft-serve ice cream to produce dry-
ness and stiffness; as a calcium ion source for reaction with alginates
to form dessert gels; and as a calcium source for food enrichment.
Definition
ChEBI: Calcium sulfate is a calcium salt and an inorganic calcium salt.
Preparation
Calcium Sulfate may be
prepared by the addition of a soluble calcium salt to
a solution of sodium sulfate:
CaCl2+ Na2SO4→CaSO4+ 2NaCl
The product is generally a dihydrate although other
hydrates are also known. The CAS numbers of these
materials are: 7778-18-9 (anhydrite); 10034-76-1 (hemihydrate); 10101-41-4 (dihydrate).
Industrial uses
Calcium sulfate (CaSO
4?2H
2O) is the common blackboard chalk.