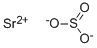Preparation
Strontium sulfite has the molecular formula of SrSO3
and the molecular weight of 167.6832 g/mol. It may be prepared by the reaction of sodiumsulfite and strontium
hydroxide:
Sr(OH)2+ Na2SO3→SrSO3+ 2NaOH
The question of the existence of hydrates remains unanswered.
Strontium sulfite occurs as colorless, crystallites
which decompose upon heating to 380°C to the oxide
plus SO2 gas. It is soluble in sulfuric acid and HCl.
There is virtually no literature concerning the physical
properties of this salt and the chemical properties
have not been delineated except in general terms.
Strontium sulfite is, however, available in limited
quantities commercially.