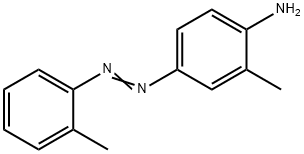
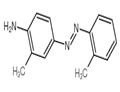
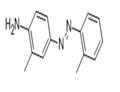
Chemical Properties
Aminoazotoluene forms golden yellow or reddish-brown crystalline solid.
Chemical Properties
red-brown crystalline powder
Uses
Coloring oils, fats and waxes; manufacture of pigments. Chemical intermediate for the production of dyes.
Preparation
(a) will Sodium nitrite (1 Moore) to join O-Methylaniline (8.5 Moore) and hydrochloric acid (1 Moore), the temperature is maintained at 28 ℃ below, until diazotization finish. Add a small amount of hydrochloric acid (about 0.2 Moore), and heating (40 ℃, 3 hours), in order to separate, with Sodium hydroxide for mercerization, and distillation reservoir. In order to get the refined products, usable alcohol recrystallization; (b) O-Methylaniline?diazotization, and O-Methylaniline?base Methanesulfonic acid coupling, and then with Sodium hydroxide solution common boiling, hydrolysis off Methanesulfonic acid base.
Definition
ChEBI: Ortho-Aminoazotoluene is a member of azobenzenes.
General Description
Reddish-brown to golden crystals; orangish red powder. Odorless.
Air & Water Reactions
Dust may form an explosive mixture in air. Insoluble in water.
Reactivity Profile
O-AMINOAZOTOLUENE is an azo compound. Azo, diazo, azido compounds can detonate. This applies in particular to organic azides that have been sensitized by the addition of metal salts or strong acids. Toxic gases are formed by mixing materials of this class with acids, aldehydes, amides, carbamates, cyanides, inorganic fluorides, halogenated organics, isocyanates, ketones, metals, nitrides, peroxides, phenols, epoxides, acyl halides, and strong oxidizing or reducing agents. Flammable gases are formed by mixing materials in this group with alkali metals. Explosive combination can occur with strong oxidizing agents, metal salts, peroxides, and sulfides. O-AMINOAZOTOLUENE is sensitive to prolonged exposure to heat. O-AMINOAZOTOLUENE is incompatible with strong oxidizing agents.
Hazard
Possible carcinogen.
Fire Hazard
Flash point data for O-AMINOAZOTOLUENE are not available. O-AMINOAZOTOLUENE is probably combustible.
Safety Profile
Confirmed carcinogen with experimental carcinogenic, neoplastigenic, and tumorigenic data. Poison by ingestion. Moderately toxic by subcutaneous route. An experimental teratogen. Human mutation data reported. When heated to decomposition it emits toxic fumes of NO,. See also AROMATIC AMINES.
Potential Exposure
An azo compound used in dyes, medicines; as a colorant in shoe polishes and other wax-based polishes.
Carcinogenicity
o-Aminoazotoluene is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.
Shipping
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Purification Methods
Recrystallise the dye twice from EtOH, once from *benzene, then dry it in an Abderhalden drying apparatus. [Cilento J Am Chem Soc 74 968 1952, Sawicki J Org Chem 21 605 1956, Beilstein 16 H 334, 16 I 322, 16 II 178, 16 III 386, 16 IV 525.] CARCINOGENIC.
Properties and Applications
yellow. In the ethanol for yellow sheet crystallization, melting point 100 ℃. Soluble in Acetone, ethanol, Cellosolve and Toluene, insoluble in water. In concentrated sulfuric acid for brown, dilution after red orange solution, and precipitation; In concentrated nitric acid for red brown solution; In concentrated hydrochloric acid partly dissolved for brown; In 10% of the sulfuric acid insoluble; In 10% Sodium hydroxide solution slightly soluble, pale yellow. Dye alcohol solution to join hydrochloric acid red hydrochloride crystal, heating the solution. Used for paraffin color.
|
Standard
|
Light Fastness
|
Heat-resistant(℃)
|
Water
|
Sodium Carbonate(5%)
|
Hydrochloric acid(5%)
|
|
Melting point
|
Stable
|
|
ISO
|
General
|
100
|
150Sublimation
|
Indissolvable
|
Indissolvable
|
Poor
|
Incompatibilities
Dust may form explosive mixture in air. Azo compounds can detonate. This applies in particular to organic azides that have been sensitized by the addition of metal salts or strong acids. Toxic gases are formed by mixing materials of this class with acids, aldehydes, amides, carbamates, cyanides, inorganic fluorides, halogenated organics, isocyanates, ketones, metals, nitrides, peroxides, phenols, epoxides, acyl halides, and strong oxidizing or reducing agents. Flammable gases are formed by mixing materials in this group with alkali metals. Explosive combination can occur with strong oxidizing agents, metal salts, peroxides, and sulfides. This chemical is sensitive to prolonged exposure to heat. This chemical is incompatible with strong oxidizing agents.