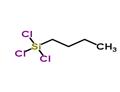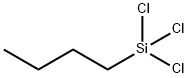
N-BUTYLTRICHLOROSILANE
- Product NameN-BUTYLTRICHLOROSILANE
- CAS7521-80-4
- CBNumberCB5705816
- MFC4H9Cl3Si
- MW191.56
- EINECS231-381-3
- MDL NumberMFCD00013605
- MOL File7521-80-4.mol
Chemical Properties
| Melting point | 147-149 °C |
| Boiling point | 149 °C(lit.) |
| Density | 1.16 g/mL at 25 °C(lit.) |
| refractive index | n |
| Flash point | 114 °F |
| form | liquid |
| Specific Gravity | 1.161 |
| color | colorless |
| Sensitive | Moisture Sensitive |
| Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents |
| BRN | 1736032 |
| CAS DataBase Reference | 7521-80-4(CAS DataBase Reference) |
| FDA UNII | ZQ9O7LYO00 |
| EPA Substance Registry System | Silane, butyltrichloro- (7521-80-4) |
| UNSPSC Code | 12352300 |
| NACRES | NA.23 |
Safety
| Symbol(GHS) |
  
|
|||||||||
| Signal word | Danger | |||||||||
| Hazard statements | H226-H302-H314 | |||||||||
| Precautionary statements | P210-P280-P301+P312+P330-P301+P330+P331-P303+P361+P353-P305+P351+P338 | |||||||||
| Hazard Codes | C | |||||||||
| Risk Statements | 10-14-34 | |||||||||
| Safety Statements | 26-36/37/39-45 | |||||||||
| RIDADR | UN 1747 8/PG 2 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | VV2080000 | |||||||||
| F | 10-21 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29319090 | |||||||||
| Hazardous Substances Data | 7521-80-4(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|