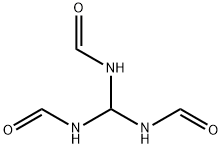
Uses
N,N′N′′-methylidynetrisformamide (TRIFO) is a source for formamide; used for N-formylformamidine (H2N-CH=N-CHO); for the N-formylformimidoyl group (H-C=NHCHO) as a building block in heterocyclic synthesis).
Preparation
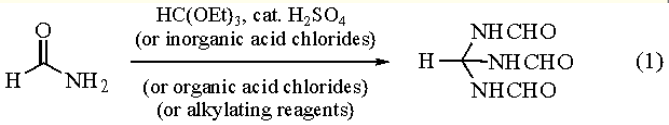
Triethyl Orthoformate reacts with formamide under catalysis of mineral acids to give TRIFO (eq
1). The reaction of excess formamide with inorganic and organic acyl chlorides such as PCl3, POCl3, SOCl2, acetyl
chloride, benzoyl chloride, and ethyl chloroformate proceeds via iminium salts to afford TRIFO. Analogously,
alkylating reagents such as dialkyl sulfates, alkyl sulfonates or triethyloxonium tetrafluoroborate transform formamide to
TRIFO. Alkoxymethyleneiminium salts and formamidinium salts are intermediates in these reactions. Pure salts of this
type are also converted to the reagent by formamide. Reaction of Dimethyl Sulfate with formamide is the easiest way to
prepare TRIFO.
General Description
N,N′,N′′-Methylidynetrisformamide is also referred as
tris(formamido)methane.
Solubility in organics
N,N′N′′-methylidynetrisformamide is soluble in formamide; soluble in aprotic dipolar solvents such as DMF or DMA on heating.
storage
N,N′N′′-methylidynetrisformamide is stored at rt in closed bottles. Use in a fume hood.