Originator
Analexin, Mallinckrodt Inc., US ,1960
Uses
Analgesic; relaxant
(skeletal muscle).
Uses
(±)-Phenyramidol can be used in biological study of preparation of nitric oxide releasing prodrugs useful in the treatment of diseases. It is also a muscle relaxant drug.
Definition
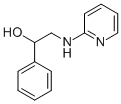
ChEBI: Fenyramidol is an aminopyridine.
Manufacturing Process
A mixture containing 188 g (0.20 mol) of 2-arninopyridine, 0.55 g of lithium amide and 75 cc of anhydrous toluene was refluxed for 1.5 hours. Styrene oxide (12.0 g = 0.10 mol) was then added to the reaction mixture with stirring over a period of ten minutes. The reaction mixture was stirred and refluxed for an additional 3.5 hours. A crystalline precipitate was formed during the reaction which was removed by filtration, MP 170°C to 171°C. 1.5 g. The filtrate was concentrated to dryness and a dark residue remained which was crystallized from anhydrous ether; yield 6.0 g. Upon recrystallization of the crude solid from 30 cc of isopropyl alcohol, 2.0 g of a light yellow solid was isolated; MP 170° to 171°C.
Therapeutic Function
Analgesic, Muscle relaxant
Synthesis Reference(s)
Journal of the American Chemical Society, 81, p. 4351, 1959
DOI: 10.1021/ja01525a062
General Description
Crystals (from dilute methanol).
Reactivity Profile
PHENYRAMIDOL is an amine and an alcohol. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides.
Fire Hazard
Flash point data for PHENYRAMIDOL are not available. PHENYRAMIDOL is probably combustible.