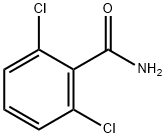
Chemical Properties
white to brown-grey crystalline powder
Uses
2,6-Dichlorobenzamide is the persistent metabolite of herbicide 2,6-Dichlorobenzonitrile (D431945).
Definition
ChEBI: A member of the class of benzamides that is benzamide substituted by chloro groups at positions 2 and 6.
Metabolic pathway
Oral doses of DCB are excreted by rats as DCB, two
monohydroxy-DCBs, 2-chloro-5-hydroxy-6-
(methylthio)benzamide, and 2-chloro-5-hydroxy-6-[S-
(N-acetyl)-cysteinyl]benzamide. Biliary excretion (33%
of the dose), enterohepatic circulation, and intestinal
microfloral metabolism are involved in the formation of
2-chloro-5-hydroxy-6-(methylthio)benzamide. The
major route for the metabolism of DCB is the
conjugation with glutathione in a process involving
phenyl ring hydroxylation at the ortho position to the
S-glutathionyl moiety. Two mechanisms can be
processed for the formation of hydroxylated
metabolites resulting from the epoxidation at the 2-
and 3-positions of the phenyl ring (for the proposed
mechanisms: see the text).