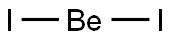Chemical Properties
-60 mesh, 99.5% pure and needles; very hygroscopic; enthalpy of vaporization 70.5 kJ/mol; enthalpy of fusion 21.00 kJ/mol; obtained by reaction of Be and I2 at 500°C–700°C [CER91] [KIR78] [MER06] [CRC10]
Preparation
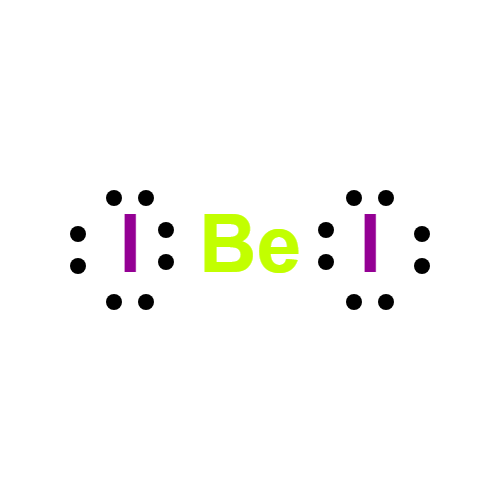
Beryllium iodide has the formula BeI2. It is very
hygroscopic and reacts violently with water, forming
hydroiodic acid. Beryllium iodide can be prepared by
reacting Be metal with elemental iodine at temperatures
of 500°C–700°C:
Be+I2→BeI2
Beryllium iodide is also formed when beryllium
carbide reacts with hydrogen iodide in the gas phase:
Be2C+4HI→2BeI2+CH4
Beryllium Iodide, BeI2, was first prepared by W?hler
(1828) and Debray (1855) who prepared the iodide by
the action of iodine upon the metal. Lebeau (1898),
was the first to prepare large quantities (for the time)
by the action of gaseous hydroiodic acid, or a mixture
of hydrogen and iodine vapor, on the carbide at about
700°C.
Beryllium iodide, as obtained in the sublimed state,
consists of colorless crystals, which are quickly decomposed
in moist air. Its specific gravity at 15°C is close to 4.20 g/cm3. It begins to sublime below its melting
point which is 510°C. The melted iodide boils between
585 and 595°C. It is insoluble in benzene, toluene, spirits
of turpentine, and but slightly soluble in carbon disulphide.
The slightest trace of water attacks it immediately,
but if it is first fused, its sensitivity to water is
much less. It can be distilled without alteration in dry
hydrogen, nitrogen or carbon dioxide. At this point in
time, the structure BeI2 has not been published.